Intravitreal Dexamethasone Implants for Refractory Macular Edema in Eyes with Noninfectious Uveitis
- PMID: 34501209
- PMCID: PMC8432099
- DOI: 10.3390/jcm10173762
Intravitreal Dexamethasone Implants for Refractory Macular Edema in Eyes with Noninfectious Uveitis
Abstract
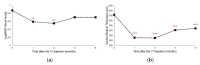
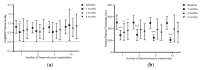
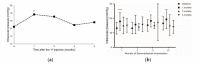
Macular edema (ME) is a common cause of visual loss among eyes with uveitis, and its management can be challenging. Steroids are an effective treatment for ME, and intravitreal dexamethasone (DEX) implants provide sustained steroid release. The purpose of this study is to evaluate intravitreal DEX implant on refractory ME in eyes with noninfectious uveitis. A retrospective study including 52 eyes of 37 patients with refractory uveitic ME was conducted from January 2011 through August 2017 at Linkou Chang Gung Memorial Hospital in Taiwan. Patients' demographic characteristics were collected. In addition, clinical information, including corrected visual acuity (VA), intraocular pressure (IOP), and central retinal thickness (CRT) on optical coherence tomography, was recorded and analyzed. During the study period, affected eyes received a total of 110 intravitreal DEX implants (range, one to six in each eye). After the first DEX implant injection in all eyes, VA significantly improved at one and two months. CRT significantly decreased one month after a single DEX implant, and the effect lasted for six months and waned over time. Patients receiving multiple DEX implants still showed significant decreases in CRT one month after the first implant. Increases in IOP were noted one month after the DEX implant, but the IOP could be medically controlled. Intravitreal DEX implants can effectively treat refractory uveitic ME, improving both VA and CRT with an acceptable safety profile. Further studies are necessary to evaluate the effect of multiple implants and long-term outcomes.
Keywords: central retinal thickness; intraocular pressure; intravitreal dexamethasone implants; refractory macular edema; visual acuity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials

