An Immunogenicity Report for the Comparison between Heterologous and Homologous Prime-Boost Schedules with ChAdOx1-S and BNT162b2 Vaccines
- PMID: 34501264
- PMCID: PMC8432244
- DOI: 10.3390/jcm10173817
An Immunogenicity Report for the Comparison between Heterologous and Homologous Prime-Boost Schedules with ChAdOx1-S and BNT162b2 Vaccines
Abstract
Background: There is a small amount of immunological data on COVID-19 heterologous vaccination schedules in humans. We assessed the immunogenicity of BNT162b2 (Pfizer/BioNTech) administered as a second dose in healthcare workers primed with ChAdOx1-S (Vaxzevria, AstraZeneca).
Methods: 197 healthcare workers were included in a monocentric observational study in Foch hospital, France, between June and July 2021. The main outcome was the immunogenicity measured by serum SARS-CoV-2 IgG antibodies.
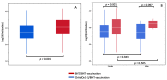
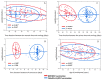
Results: 130 participants received the ChAdOx1-S/BNT vaccine and 67 received the BNT/BNT vaccine. The geometric mean of IgG antibodies was significantly higher in the BNT/BNT vaccine group compared to the ChAdOx1-S/BNT vaccine group, namely 10,734.9, 95% CI (9141.1-12,589.3) vs. 7268.6, 95% CI (6501.3-8128.3), respectively (p < 0.001). However, after adjustment for time duration between the prime and second vaccinations, no significant difference was observed (p = 0.181). A negative correlation between antibody levels and time duration between second dose and serology test was observed for the BNT/BNT vaccine (p < 0.001), which remained significant after adjustment for all covariates (p < 0.001), but not for the ChAdOx1-S/BNT vaccine (p = 0.467).
Conclusions: Heterologous and homologous schedules of ChAdOx1-S and BNT vaccines present robust immune responses after the second vaccination. The results observed were equivalent after adjustment for covariates and emphasize the importance of flexibility in deploying mRNA and viral vectored vaccines. Nevertheless, applying the ChAdOx1-S schedule vaccination for the heterologous second dose of BNT was associated with decreased IgG antibody levels compared to the homologous BNT/BNT vaccination.
Keywords: BNT162b2; COVID-19; COVID-19 vaccine; ChAdOx1-S; immunogenicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing Antibody Levels Are Highly Predictive of Immune Protection from Symptomatic SARS-CoV-2 Infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. - DOI - PubMed
-
- Vallée A., Chan-Hew-Wai A., Bonan B., Lesprit P., Parquin F., Catherinot É., Choucair J., Billard D., Amiel-Taieb C., Camps È., et al. Oxford-AstraZeneca COVID-19 Vaccine: Need of a Reasoned and Effective Vaccine Campaign. Public Health. 2021;196:135–137. doi: 10.1016/j.puhe.2021.05.030. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

