Small Neuron-Derived Extracellular Vesicles from Individuals with Down Syndrome Propagate Tau Pathology in the Wildtype Mouse Brain
- PMID: 34501378
- PMCID: PMC8432237
- DOI: 10.3390/jcm10173931
Small Neuron-Derived Extracellular Vesicles from Individuals with Down Syndrome Propagate Tau Pathology in the Wildtype Mouse Brain
Abstract
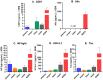
Individuals with Down syndrome (DS) exhibit Alzheimer's disease (AD) pathology at a young age, including amyloid plaques and neurofibrillary tangles (NFTs). Tau pathology can spread via extracellular vesicles, such as exosomes. The cargo of neuron-derived small extracellular vesicles (NDEVs) from individuals with DS contains p-Tau at an early age. The goal of the study was to investigate whether NDEVs isolated from the blood of individuals with DS can spread Tau pathology in the brain of wildtype mice. We purified NDEVs from the plasma of patients with DS-AD and controls and injected small quantities using stereotaxic surgery into the dorsal hippocampus of adult wildtype mice. Seeding competent Tau conformers were amplified in vitro from DS-AD NDEVs but not NDEVs from controls. One month or 4 months post-injection, we examined Tau pathology in mouse brains. We found abundant p-Tau immunostaining in the hippocampus of the mice injected with DS-AD NDEVs compared to injections of age-matched control NDEVs. Double labeling with neuronal and glial markers showed that p-Tau staining was largely found in neurons and, to a lesser extent, in glial cells and that p-Tau immunostaining was spreading along the corpus callosum and the medio-lateral axis of the hippocampus. These studies demonstrate that NDEVs from DS-AD patients exhibit Tau seeding capacity and give rise to tangle-like intracellular inclusions.
Keywords: Alzheimer’s disease; Down syndrome; aging; biomarkers; neuropathology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Capone G.T., Chicoine B., Bulova P., Stephens M., Hart S., Crissman B., Videlefsky A., Myers K., Roizen N., Esbensen A., et al. Co-occurring medical conditions in adults with Down syndrome: A systematic review toward the development of health care guidelines. Am. J. Med. Genet. A. 2018;176:116–133. doi: 10.1002/ajmg.a.38512. - DOI - PubMed
-
- Delabar J.M., Allinquant B., Bianchi D., Blumenthal T., Dekker A., Edgin J., O’Bryan J., Dierssen M., Potier M.C., Wiseman F., et al. Changing Paradigms in Down Syndrome: The First International Conference of the Trisomy 21 Research Society. Mol. Syndr. 2016;7:251–261. doi: 10.1159/000449049. - DOI - PMC - PubMed
-
- Egensperger R., Weggen S., Ida N., Multhaup G., Schnabel R., Beyreuther K., Bayer T.A. Reverse relationship between beta-amyloid precursor protein and beta-amyloid peptide plaques in Down’s syndrome versus sporadic/familial Alzheimer’s disease. Acta Neuropathol. 1999;97:113–118. doi: 10.1007/s004010050963. - DOI - PubMed
-
- Fortea J., Carmona-Iragui M., Benejam B., Fernandez S., Videla L., Barroeta I., Alcolea D., Pegueroles J., Munoz L., Belbin O., et al. Plasma and CSF biomarkers for the diagnosis of Alzheimer’s disease in adults with Down syndrome: A cross-sectional study. Lancet Neurol. 2018;17:860–869. doi: 10.1016/S1474-4422(18)30285-0. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

