Imaging of Pancreatic Neuroendocrine Neoplasms
- PMID: 34501485
- PMCID: PMC8430610
- DOI: 10.3390/ijerph18178895
Imaging of Pancreatic Neuroendocrine Neoplasms
Abstract
Pancreatic neuroendocrine neoplasms (panNENs) represent the second most common pancreatic tumors. They are a heterogeneous group of neoplasms with varying clinical expression and biological behavior, from indolent to aggressive ones. PanNENs can be functioning or non-functioning in accordance with their ability or not to produce metabolically active hormones. They are histopathologically classified according to the 2017 World Health Organization (WHO) classification system. Although the final diagnosis of neuroendocrine tumor relies on histologic examination of biopsy or surgical specimens, both morphologic and functional imaging are crucial for patient care. Morphologic imaging with ultrasonography (US), computed tomography (CT) and magnetic resonance imaging (MRI) is used for initial evaluation and staging of disease, as well as surveillance and therapy monitoring. Functional imaging techniques with somatostatin receptor scintigraphy (SRS) and positron emission tomography (PET) are used for functional and metabolic assessment that is helpful for therapy management and post-therapeutic re-staging. This article reviews the morphological and functional imaging modalities now available and the imaging features of panNENs. Finally, future imaging challenges, such as radiomics analysis, are illustrated.
Keywords: abdominal radiology; computed tomography (CT); gastrointestinal radiology; magnetic resonance imaging (MRI); pancreatic neuroendocrine neoplasms (panNENs); positron emission tomography (PET); somatostatin receptor scintigraphy (SRS).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
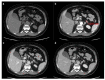



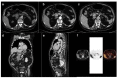
References
-
- Bilimoria K.Y., Tomlinson J.S., Merkow R.P., Stewart A.K., Ko C.Y., Talamonti M.S., Bentrem D.J. Clinicopathologic features and treatment trends of pancreatic neuroendocrine tumors: Analysis of 9,821 patients. J. Gastrointest. Surg. 2007;11:1460–1467; discussion 1467–1469. doi: 10.1007/s11605-007-0263-3. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

