Is It Possible to Have Home E-Monitoring of Pulmonary Function in Our Patients with Duchenne Muscular Dystrophy in the COVID-19 Pandemic?-A One Center Pilot Study
- PMID: 34501557
- PMCID: PMC8430665
- DOI: 10.3390/ijerph18178967
Is It Possible to Have Home E-Monitoring of Pulmonary Function in Our Patients with Duchenne Muscular Dystrophy in the COVID-19 Pandemic?-A One Center Pilot Study
Abstract
Background: Duchenne muscular dystrophy (DMD) is the most common, progressive, irreversible muscular dystrophy. Pulmonary function is crucial for duration of life in this disease. Currently, the European Respiratory Society is focused on digital health, seeking innovations that will be realistic for digital respiratory medicine to support professionals and patients during the COVID-19 pandemic.
Aims: The aim of this study was to investigate whether it is possible to monitor pulmonary function at home using an individual electronic spirometry system in boys with Duchenne muscular dystrophy.
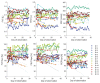
Materials and methods: In this observational, prospective study, conducted from March 2021 to June 2021, twenty boys with DMD (aged 8-16) were enrolled. The patients were recruited from the Rare Disease Centre, University Clinical Centre, of Gdańsk, Poland. Medical history and anthropometric data were collected, and spirometry (Jaeger, Germany) was performed in all patients at the start of the study. Each patient received an electronic individual spirometer (AioCare) and was asked to perform spirometry on their own every day, morning and evening, at home for a period of 4 weeks. The number of measurements, correctness of performing measurements, forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), and peak expiratory flow (PEF) were evaluated.
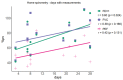
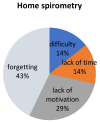
Results: Finally, 14 out of 20 boys enrolled in the study with a mean age of 12.5 years (7 non-ambulatory) applied and received a home spirometer (AioCare). A total of 283 measurements were performed by all patients at home for 4 weeks. Half of the patients were able to perform measurements correctly. There were no significant differences between mean values of FVC, FE1, PEF between home and hospital spirometry (p > 0.05) expect PEF pv% (p < 0.00046). Patients with higher FEV1 (p = 0.0387) and lower BMI (p = 0.0494) were more likely to take home spirometer measurements. The mean general satisfaction rating of home-spirometry was 4.33/5 (SD 0.78), the mean intelligibility rating was 4.83/5 (SD 0.58). Reasons for irregular measurements were: forgetting (43%), lack of motivation (29%), difficulty (14%), lack of time (14%).
Conclusion: Home electronic monitoring of pulmonary function in patients with DMD is possible to implement in daily routines at home. This protocol should be introduced as early as possible in patients 7-8 years old with good, preserved lung function. Patients accept this form of medical care but require more education about the benefits of e-monitoring. There is a need to implement a system to remind patients of the use of electronic medical devices at home, e.g., via SMS (short message service).
Keywords: AioCare; COVID-19; Duchenne muscular dystrophy; digital health; e-monitoring of pulmonary function; home monitoring; home monitoring pulmonary function; pulmonary function test; rare diseases; spirometry.
Conflict of interest statement
E.W., A.S.-R., S.M., D.S., and E.J. declare no conflict of interest, M.S. is employed by HealthUp.
Figures
References
-
- Birnkrant D.J., Bushby K., Bann C.M., Apkon S.D., Blackwell A., Brumbaugh D., Case E.L., Clemens P.R., Hadjiyannakis S., Pandya S., et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018;17:251–267. doi: 10.1016/S1474-4422(18)30024-3. - DOI - PMC - PubMed
-
- Birnkrant D.J., Bushby K., Bann C., Alman A.B., Apkon S.D., Blackwell A., Case L., Cripe L., Hadjiyannakis S., Olson A.K., et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: Respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018;17:347–361. doi: 10.1016/S1474-4422(18)30025-5. - DOI - PMC - PubMed
-
- Wasilewska E., Małgorzewicz S., Meyer-Szary J., Sledzinska K., Niedoszytko M.B., Jassem E., Wierzba J. Pulmonary dysfunction in children with Duchenne muscular dystrophy may appear earlier than we thought—Analysis using novel methodology based on z-scores. Arch. Med. Sci. 2021 doi: 10.5114/aoms/119782. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

