Properties and Application of Cell-Free DNA as a Clinical Biomarker
- PMID: 34502023
- PMCID: PMC8431421
- DOI: 10.3390/ijms22179110
Properties and Application of Cell-Free DNA as a Clinical Biomarker
Abstract
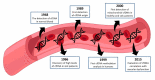
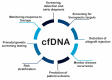
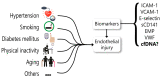
Biomarkers are valuable tools in clinical practice. In 2001, the National Institutes of Health (NIH) standardized the definition of a biomarker as a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacological responses to a therapeutic intervention. A biomarker has clinical relevance when it presents precision, standardization and reproducibility, suitability to the patient, straightforward interpretation by clinicians, and high sensitivity and/or specificity by the parameter it proposes to identify. Thus, serum biomarkers should have advantages related to the simplicity of the procedures and to the fact that venous blood collection is commonplace in clinical practice. We described the potentiality of cfDNA as a general clinical biomarker and focused on endothelial dysfunction. Circulating cell-free DNA (cfDNA) refers to extracellular DNA present in body fluid that may be derived from both normal and diseased cells. An increasing number of studies demonstrate the potential use of cfDNA as a noninvasive biomarker to determine physiologic and pathologic conditions. However, although still scarce, increasing evidence has been reported regarding using cfDNA in cardiovascular diseases. Here, we have reviewed the history of cfDNA, its source, molecular features, and release mechanism. We also show recent studies that have investigated cfDNA as a possible marker of endothelial damage in clinical settings. In the cardiovascular system, the studies are quite new, and although interesting, stronger evidence is still needed. However, some drawbacks in cfDNA methodologies should be overcome before its recommendation as a biomarker in the clinical setting.
Keywords: biomarker; cfDNA; circulating nucleotide; endothelial dysfunction; vascular damage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Characteristics, properties, and potential applications of circulating cell-free dna in clinical diagnostics: a focus on transplantation.J Immunol Methods. 2018 Dec;463:27-38. doi: 10.1016/j.jim.2018.09.011. Epub 2018 Sep 26. J Immunol Methods. 2018. PMID: 30267663 Review.
-
Endotheliopathy is associated with higher levels of cell-free DNA following major trauma: A prospective observational study.PLoS One. 2017 Dec 19;12(12):e0189870. doi: 10.1371/journal.pone.0189870. eCollection 2017. PLoS One. 2017. PMID: 29261771 Free PMC article.
-
Cell-free DNA as a biomarker in stroke: Current status, problems and perspectives.Crit Rev Clin Lab Sci. 2018 Jan;55(1):55-70. doi: 10.1080/10408363.2017.1420032. Epub 2018 Jan 5. Crit Rev Clin Lab Sci. 2018. PMID: 29303618 Review.
-
Technical Advances in Circulating Cell-Free DNA Detection and Analysis for Personalized Medicine in Patients' Care.Biomolecules. 2024 Apr 19;14(4):498. doi: 10.3390/biom14040498. Biomolecules. 2024. PMID: 38672514 Free PMC article. Review.
-
Cell free DNA as a diagnostic and prognostic marker for cardiovascular diseases.Clin Chim Acta. 2020 Apr;503:145-150. doi: 10.1016/j.cca.2020.01.013. Epub 2020 Jan 21. Clin Chim Acta. 2020. PMID: 31978408 Free PMC article. Review.
Cited by
-
Neutrophils and the Systemic Inflammatory Response Syndrome (SIRS).Int J Mol Sci. 2023 Aug 30;24(17):13469. doi: 10.3390/ijms241713469. Int J Mol Sci. 2023. PMID: 37686271 Free PMC article. Review.
-
The Efficacy of Liquid Biopsy of Total cfDNA for Predicting Systemic Metastasis in Japanese Patients With Oral Squamous Cell Carcinoma.Head Neck. 2025 May;47(5):1411-1420. doi: 10.1002/hed.28054. Epub 2024 Dec 27. Head Neck. 2025. PMID: 39731308 Free PMC article.
-
Infective Endocarditis during Pregnancy-Keep It Safe and Simple!Medicina (Kaunas). 2023 May 12;59(5):939. doi: 10.3390/medicina59050939. Medicina (Kaunas). 2023. PMID: 37241171 Free PMC article. Review.
-
Deciphering the interplay: circulating cell-free DNA, signaling pathways, and disease progression in idiopathic pulmonary fibrosis.3 Biotech. 2025 Apr;15(4):102. doi: 10.1007/s13205-025-04272-y. Epub 2025 Mar 29. 3 Biotech. 2025. PMID: 40165930 Review.
-
Profiling of cell-free DNA methylation and histone signatures in pediatric NAFLD: A pilot study.Hepatol Commun. 2022 Dec;6(12):3311-3323. doi: 10.1002/hep4.2082. Epub 2022 Oct 20. Hepatol Commun. 2022. PMID: 36264206 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

