Gene Therapy for Neuronopathic Mucopolysaccharidoses: State of the Art
- PMID: 34502108
- PMCID: PMC8430935
- DOI: 10.3390/ijms22179200
Gene Therapy for Neuronopathic Mucopolysaccharidoses: State of the Art
Abstract
The need for long-lasting and transformative therapies for mucopolysaccharidoses (MPS) cannot be understated. Currently, many forms of MPS lack a specific treatment and in other cases available therapies, such as enzyme replacement therapy (ERT), do not reach important areas such as the central nervous system (CNS). The advent of newborn screening procedures represents a major step forward in early identification and treatment of individuals with MPS. However, the treatment of brain disease in neuronopathic MPS has been a major challenge to date, mainly because the blood brain barrier (BBB) prevents penetration of the brain by large molecules, including enzymes. Over the last years several novel experimental therapies for neuronopathic MPS have been investigated. Gene therapy and gene editing constitute potentially curative treatments. However, despite recent progress in the field, several considerations should be taken into account. This review focuses on the state of the art of in vivo and ex vivo gene therapy-based approaches targeting the CNS in neuronopathic MPS, discusses clinical trials conducted to date, and provides a vision for the future implications of these therapies for the medical community. Recent advances in the field, as well as limitations relating to efficacy, potential toxicity, and immunogenicity, are also discussed.
Keywords: adeno-associated virus; blood brain barrier; central nervous system; gene therapy; lentivirus; mucopolysaccharidoses; viral vectors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
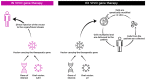
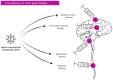
References
-
- Scarpa M., Almássy Z., Beck M., Bodamer O., Bruce I.A., De Meirleir L., Guffon N., Guillén-Navarro E., Hensman P., Jones S., et al. Hunter Syndrome European Expert Council.Mucopolysaccharidosis type II: European recommendations for the diagnosis and multidisciplinary management of a rare disease. Orphanet. J. Rare. Dis. 2011;6:72. doi: 10.1186/1750-1172-6-72. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

