Tau Protein Interaction Partners and Their Roles in Alzheimer's Disease and Other Tauopathies
- PMID: 34502116
- PMCID: PMC8431036
- DOI: 10.3390/ijms22179207
Tau Protein Interaction Partners and Their Roles in Alzheimer's Disease and Other Tauopathies
Abstract
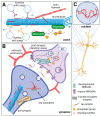
Tau protein plays a critical role in the assembly, stabilization, and modulation of microtubules, which are important for the normal function of neurons and the brain. In diseased conditions, several pathological modifications of tau protein manifest. These changes lead to tau protein aggregation and the formation of paired helical filaments (PHF) and neurofibrillary tangles (NFT), which are common hallmarks of Alzheimer's disease and other tauopathies. The accumulation of PHFs and NFTs results in impairment of physiological functions, apoptosis, and neuronal loss, which is reflected as cognitive impairment, and in the late stages of the disease, leads to death. The causes of this pathological transformation of tau protein haven't been fully understood yet. In both physiological and pathological conditions, tau interacts with several proteins which maintain their proper function or can participate in their pathological modifications. Interaction partners of tau protein and associated molecular pathways can either initiate and drive the tau pathology or can act neuroprotective, by reducing pathological tau proteins or inflammation. In this review, we focus on the tau as a multifunctional protein and its known interacting partners active in regulations of different processes and the roles of these proteins in Alzheimer's disease and tauopathies.
Keywords: Alzheimer’s disease; interaction partners; tau protein; tauopathies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Uversky V.N. Intrinsic disorder, protein–protein interactions, and disease. Adv. Protein Chem. Struct. Biol. 2018;110:85–121. - PubMed
-
- Ferreira A., Busciglio J., Cáceres A. Microtubule formation and neurite growth in cerebellar macroneurons which develop in vitro: Evidence for the involvement of the microtubule-associated proteins, MAP-1a, HMW-MAP2 and Tau. Dev. Brain Res. 1989;49:215–228. doi: 10.1016/0165-3806(89)90023-0. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

