Nanoarchitectonics: Complexes and Conjugates of Platinum Drugs with Silicon Containing Nanocarriers. An Overview
- PMID: 34502173
- PMCID: PMC8430569
- DOI: 10.3390/ijms22179264
Nanoarchitectonics: Complexes and Conjugates of Platinum Drugs with Silicon Containing Nanocarriers. An Overview
Abstract
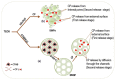
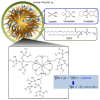
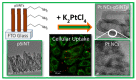
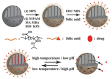
The development in the area of novel anticancer prodrugs (conjugates and complexes) has attracted growing attention from many research groups. The dangerous side effects of currently used anticancer drugs, including cisplatin and other platinum based drugs, as well their systemic toxicity is a driving force for intensive search and presents a safer way in delivery platform of active molecules. Silicon based nanocarriers play an important role in achieving the goal of synthesis of the more effective prodrugs. It is worth to underline that silicon based platform including silica and silsesquioxane nanocarriers offers higher stability, biocompatibility of such the materials and pro-longed release of active platinum drugs. Silicon nanomaterials themselves are well-known for improving drug delivery, being themselves non-toxic, and versatile, and tailored surface chemistry. This review summarizes the current state-of-the-art within constructs of silicon-containing nano-carriers conjugated and complexed with platinum based drugs. Contrary to a number of other reviews, it stresses the role of nano-chemistry as a primary tool in the development of novel prodrugs.
Keywords: anticancer platinum drugs; nanocomplexes synthesis and properties; nanoconjugates; silicon containing nanocarriers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



























References
-
- Kumar C.G., Poornachandra Y., Pombala S. Micro and Nano Technologies. Elsevier Inc.; Amsterdam, The Netherlands: 2017. pp. 1–61.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

