Protective and Anti-Inflammatory Effects of Protegrin-1 on Citrobacter rodentium Intestinal Infection in Mice
- PMID: 34502403
- PMCID: PMC8431371
- DOI: 10.3390/ijms22179494
Protective and Anti-Inflammatory Effects of Protegrin-1 on Citrobacter rodentium Intestinal Infection in Mice
Abstract
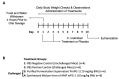
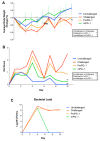
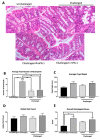
Infectious intestinal colitis, manifesting as intestinal inflammation, diarrhea, and epithelial barrier disruption, affects millions of humans worldwide and, without effective treatment, can result in death. In addition to this, the significant rise in antibiotic-resistant bacteria poses an urgent need for alternative anti-infection therapies for the treatment of intestinal disorders. Antimicrobial peptides (AMPs) are potential therapies that have broad-spectrum antimicrobial activity due to their (1) unique mode of action, (2) broad-spectrum antimicrobial activity, and (3) protective role in GI tract maintenance. Protegrin-1 (PG-1) is an AMP of pig origin that was previously shown to reduce the pathological effects of chemically induced digestive tract inflammation (colitis) and to modulate immune responses and tissue repair. This study aimed to extend these findings by investigating the protective effects of PG-1 on pathogen-induced colitis in an infection study over a 10-day experimental period. The oral administration of PG-1 reduced Citrobacter rodentium intestinal infection in mice as evidenced by reduced histopathologic change in the colon, prevention of body weight loss, milder clinical signs of disease, and more effective clearance of bacterial infection relative to challenged phosphate-buffered saline (PBS)-treated mice. Additionally, PG-1 treatment altered the expression of various inflammatory mediators during infection, which may act to resolve inflammation and re-establish intestinal homeostasis. PG-1 administered in its mature form was more effective relative to the pro-form (ProPG-1). To our knowledge, this is the first study demonstrating the protective effects of PG-1 on infectious colitis.
Keywords: Citrobacter rodentium infection; antimicrobial peptide; immunomodulatory effect; infectious colitis; protegrin-1.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that there was no conflict of interest in this study.
Figures

Similar articles
-
Dietary vitamin D3 deficiency alters intestinal mucosal defense and increases susceptibility to Citrobacter rodentium-induced colitis.Am J Physiol Gastrointest Liver Physiol. 2015 Nov 1;309(9):G730-42. doi: 10.1152/ajpgi.00006.2015. Epub 2015 Sep 3. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 26336925 Free PMC article.
-
Effects of hypoxic exposure on immune responses of intestinal mucosa to Citrobacter colitis in mice.Biomed Pharmacother. 2020 Sep;129:110477. doi: 10.1016/j.biopha.2020.110477. Epub 2020 Jul 6. Biomed Pharmacother. 2020. PMID: 32768962
-
Vasoactive intestinal peptide ameliorates intestinal barrier disruption associated with Citrobacter rodentium-induced colitis.Am J Physiol Gastrointest Liver Physiol. 2009 Oct;297(4):G735-50. doi: 10.1152/ajpgi.90551.2008. Epub 2009 Aug 6. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19661153
-
T cell subsets and environmental factors in Citrobacter rodentium infection.Curr Opin Microbiol. 2021 Oct;63:92-97. doi: 10.1016/j.mib.2021.06.006. Epub 2021 Jul 21. Curr Opin Microbiol. 2021. PMID: 34298480 Review.
-
Prevention and potential repair of colitis: Beneficial effects and regulatory mechanisms of food-derived anti-inflammatory peptides.Crit Rev Food Sci Nutr. 2024;64(23):8184-8202. doi: 10.1080/10408398.2023.2197068. Epub 2023 Apr 5. Crit Rev Food Sci Nutr. 2024. PMID: 37017113 Review.
Cited by
-
Polysaccharides from Pseudostellaria heterophylla modulate gut microbiota and alleviate syndrome of spleen deficiency in rats.Sci Rep. 2022 Nov 23;12(1):20217. doi: 10.1038/s41598-022-24329-9. Sci Rep. 2022. PMID: 36418343 Free PMC article.
-
The Addition of a Synthetic LPS-Targeting Domain Improves Serum Stability While Maintaining Antimicrobial, Antibiofilm, and Cell Stimulating Properties of an Antimicrobial Peptide.Biomolecules. 2020 Jul 8;10(7):1014. doi: 10.3390/biom10071014. Biomolecules. 2020. PMID: 32650576 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

