Failure of Diphtheria Toxin Model to Induce Parkinson-Like Behavior in Mice
- PMID: 34502404
- PMCID: PMC8430633
- DOI: 10.3390/ijms22179496
Failure of Diphtheria Toxin Model to Induce Parkinson-Like Behavior in Mice
Abstract
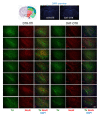
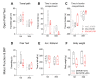
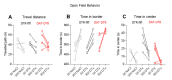
Rodent models of Parkinson's disease are based on transgenic expression of mutant synuclein, deletion of PD genes, injections of MPTP or rotenone, or seeding of synuclein fibrils. The models show histopathologic features of PD such as Lewi bodies but mostly only subtle in vivo manifestations or systemic toxicity. The models only partly mimic a predominant loss of dopaminergic neurons in the substantia nigra. We therefore generated mice that express the transgenic diphtheria toxin receptor (DTR) specifically in DA neurons by crossing DAT-Cre mice with Rosa26 loxP-STOP-loxP DTR mice. After defining a well-tolerated DTx dose, DAT-DTR and DTR-flfl controls were subjected to non-toxic DTx treatment (5 × 100 pg/g) and subsequent histology and behavioral tests. DAT protein levels were reduced in the midbrain, and tyrosine hydroxylase-positive neurons were reduced in the substantia nigra, whereas the pan-neuronal marker NeuN was not affected. Despite the promising histologic results, there was no difference in motor function tests or open field behavior. These are tests in which double mutant Pink1-/-SNCAA53T Parkinson mice show behavioral abnormalities. Higher doses of DTx were toxic in both groups. The data suggest that DTx treatment in mice with Cre/loxP-driven DAT-DTR expression leads to partial ablation of DA-neurons but without PD-reminiscent behavioral correlates.
Keywords: Cre-recombinase; SLC6a3; diphtheria toxin; dopamine transporter; motor functions; open field behavior; tyrosine hydroxylase.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
AAV1/2-induced overexpression of A53T-α-synuclein in the substantia nigra results in degeneration of the nigrostriatal system with Lewy-like pathology and motor impairment: a new mouse model for Parkinson's disease.Acta Neuropathol Commun. 2017 Feb 1;5(1):11. doi: 10.1186/s40478-017-0416-x. Acta Neuropathol Commun. 2017. PMID: 28143577 Free PMC article.
-
Exercise in an animal model of Parkinson's disease: Motor recovery but not restoration of the nigrostriatal pathway.Neuroscience. 2017 Sep 17;359:224-247. doi: 10.1016/j.neuroscience.2017.07.031. Epub 2017 Jul 25. Neuroscience. 2017. PMID: 28754312
-
Intracerebral Administration of a Ligand-ASO Conjugate Selectively Reduces α-Synuclein Accumulation in Monoamine Neurons of Double Mutant Human A30P*A53T*α-Synuclein Transgenic Mice.Int J Mol Sci. 2021 Mar 13;22(6):2939. doi: 10.3390/ijms22062939. Int J Mol Sci. 2021. PMID: 33805843 Free PMC article.
-
alpha-Synuclein- and MPTP-generated rodent models of Parkinson's disease and the study of extracellular striatal dopamine dynamics: a microdialysis approach.CNS Neurol Disord Drug Targets. 2010 Aug;9(4):482-90. doi: 10.2174/187152710791556177. CNS Neurol Disord Drug Targets. 2010. PMID: 20522009 Review.
-
Reprint of: revisiting oxidative stress and mitochondrial dysfunction in the pathogenesis of Parkinson disease-resemblance to the effect of amphetamine drugs of abuse.Free Radic Biol Med. 2013 Sep;62:186-201. doi: 10.1016/j.freeradbiomed.2013.05.042. Epub 2013 Jun 3. Free Radic Biol Med. 2013. PMID: 23743292 Review.
Cited by
-
Enhanced perceptual task performance without deprivation in mice using medial forebrain bundle stimulation.Cell Rep Methods. 2022 Dec 2;2(12):100355. doi: 10.1016/j.crmeth.2022.100355. eCollection 2022 Dec 19. Cell Rep Methods. 2022. PMID: 36590697 Free PMC article.
-
Exendin-4(1-32)K-Capric Acid, a Glucagon-Like Peptide-1 Receptor Agonist, Suppresses Food Intake via Arcuate Pro-Opiomelanocortin Neurons.Endocrinol Metab (Seoul). 2025 Jun;40(3):434-447. doi: 10.3803/EnM.2024.2185. Epub 2025 Apr 14. Endocrinol Metab (Seoul). 2025. PMID: 40223290 Free PMC article.
-
AgRP neurons are not indispensable for body weight maintenance in adult mice.Cell Rep. 2023 Jul 25;42(7):112789. doi: 10.1016/j.celrep.2023.112789. Epub 2023 Jul 8. Cell Rep. 2023. PMID: 37422762 Free PMC article.
References
-
- Paumier K.L., Sukoff Rizzo S.J., Berger Z., Chen Y., Gonzales C., Kaftan E., Li L., Lotarski S., Monaghan M., Shen W., et al. Behavioral characterization of a53t mice reveals early and late stage deficits related to parkinson’s disease. PLoS ONE. 2013;8:e70274. doi: 10.1371/journal.pone.0070274. - DOI - PMC - PubMed
-
- Gispert S., Del Turco D., Garrett L., Chen A., Bernard D.J., Hamm-Clement J., Korf H.W., Deller T., Braak H., Auburger G., et al. Transgenic mice expressing mutant a53t human alpha-synuclein show neuronal dysfunction in the absence of aggregate formation. Mol. Cell. Neurosci. 2003;24:419–429. doi: 10.1016/S1044-7431(03)00198-2. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

