Neddylation Regulates Class IIa and III Histone Deacetylases to Mediate Myoblast Differentiation
- PMID: 34502418
- PMCID: PMC8431717
- DOI: 10.3390/ijms22179509
Neddylation Regulates Class IIa and III Histone Deacetylases to Mediate Myoblast Differentiation
Abstract
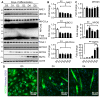
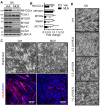
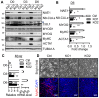
As the largest tissue in the body, skeletal muscle has multiple functions in movement and energy metabolism. Skeletal myogenesis is controlled by a transcriptional cascade including a set of muscle regulatory factors (MRFs) that includes Myogenic Differentiation 1 (MYOD1), Myocyte Enhancer Factor 2 (MEF2), and Myogenin (MYOG), which direct the fusion of myogenic myoblasts into multinucleated myotubes. Neddylation is a posttranslational modification that covalently conjugates ubiquitin-like NEDD8 (neural precursor cell expressed, developmentally downregulated 8) to protein targets. Inhibition of neddylation impairs muscle differentiation; however, the underlying molecular mechanisms remain less explored. Here, we report that neddylation is temporally regulated during myoblast differentiation. Inhibition of neddylation through pharmacological blockade using MLN4924 (Pevonedistat) or genetic deletion of NEDD8 Activating Enzyme E1 Subunit 1 (NAE1), a subunit of the E1 neddylation-activating enzyme, blocks terminal myoblast differentiation partially through repressing MYOG expression. Mechanistically, we found that neddylation deficiency enhances the mRNA and protein expressions of class IIa histone deacetylases 4 and 5 (HDAC4 and 5) and prevents the downregulation and nuclear export of class III HDAC (NAD-Dependent Protein Deacetylase Sirtuin-1, SIRT1), all of which have been shown to repress MYOD1-mediated MYOG transcriptional activation. Together, our findings for the first time identify the crucial role of neddylation in mediating class IIa and III HDAC co-repressors to control myogenic program and provide new insights into the mechanisms of muscle disease and regeneration.
Keywords: histone deacetylases; myoblast differentiation; neddylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Ubiquitin-specific protease 4 (USP4) suppresses myoblast differentiation by down regulating MyoD activity in a catalytic-independent manner.Cell Signal. 2017 Jul;35:48-60. doi: 10.1016/j.cellsig.2017.03.008. Epub 2017 Mar 21. Cell Signal. 2017. PMID: 28336234
-
Mirk/dyrk1B decreases the nuclear accumulation of class II histone deacetylases during skeletal muscle differentiation.J Biol Chem. 2005 Feb 11;280(6):4894-905. doi: 10.1074/jbc.M411894200. Epub 2004 Nov 16. J Biol Chem. 2005. PMID: 15546868
-
c-Ski activates MyoD in the nucleus of myoblastic cells through suppression of histone deacetylases.Genes Cells. 2007 Mar;12(3):375-85. doi: 10.1111/j.1365-2443.2007.01052.x. Genes Cells. 2007. PMID: 17352741
-
A novel approach to explore metabolic diseases: Neddylation.Pharmacol Res. 2024 Dec;210:107532. doi: 10.1016/j.phrs.2024.107532. Epub 2024 Dec 3. Pharmacol Res. 2024. PMID: 39637955 Review.
-
Diverse and pivotal roles of neddylation in metabolism and immunity.FEBS J. 2021 Jul;288(13):3884-3912. doi: 10.1111/febs.15584. Epub 2020 Oct 20. FEBS J. 2021. PMID: 33025631 Review.
Cited by
-
MYOD-SKP2 axis boosts tumorigenesis in fusion negative rhabdomyosarcoma by preventing differentiation through p57Kip2 targeting.Nat Commun. 2023 Dec 15;14(1):8373. doi: 10.1038/s41467-023-44130-0. Nat Commun. 2023. PMID: 38102140 Free PMC article.
-
Neddylation and Its Target Cullin 3 Are Essential for Adipocyte Differentiation.Cells. 2024 Oct 5;13(19):1654. doi: 10.3390/cells13191654. Cells. 2024. PMID: 39404417 Free PMC article.
-
MLN4924 Promotes Self-Renewal of Limbal Stem Cells and Ocular Surface Restoration.J Pers Med. 2023 Feb 21;13(3):379. doi: 10.3390/jpm13030379. J Pers Med. 2023. PMID: 36983561 Free PMC article.
-
Dietary Leucine Supplementation Improves Muscle Fiber Growth and Development by Activating AMPK/Sirt1 Pathway in Blunt Snout Bream (Megalobrama amblycephala).Aquac Nutr. 2022 Dec 21;2022:7285851. doi: 10.1155/2022/7285851. eCollection 2022. Aquac Nutr. 2022. PMID: 36860449 Free PMC article.
-
Molecular Research on Muscle Protein and Myopathies.Int J Mol Sci. 2022 Jun 26;23(13):7098. doi: 10.3390/ijms23137098. Int J Mol Sci. 2022. PMID: 35806104 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

