Alisporivir Treatment Alleviates Mitochondrial Dysfunction in the Skeletal Muscles of C57BL/6NCrl Mice with High-Fat Diet/Streptozotocin-Induced Diabetes Mellitus
- PMID: 34502433
- PMCID: PMC8430760
- DOI: 10.3390/ijms22179524
Alisporivir Treatment Alleviates Mitochondrial Dysfunction in the Skeletal Muscles of C57BL/6NCrl Mice with High-Fat Diet/Streptozotocin-Induced Diabetes Mellitus
Erratum in
-
Correction: Belosludtsev et al. Alisporivir Treatment Alleviates Mitochondrial Dysfunction in the Skeletal Muscles of C57BL/6NCrl Mice with High-Fat Diet/Streptozotocin-Induced Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 9524.Int J Mol Sci. 2024 Nov 11;25(22):12080. doi: 10.3390/ijms252212080. Int J Mol Sci. 2024. PMID: 39596548 Free PMC article.
Abstract
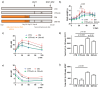
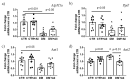
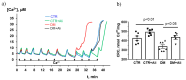
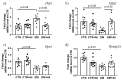
Diabetes mellitus is a systemic metabolic disorder associated with mitochondrial dysfunction, with mitochondrial permeability transition (MPT) pore opening being recognized as one of its pathogenic mechanisms. Alisporivir has been recently identified as a non-immunosuppressive analogue of the MPT pore blocker cyclosporin A and has broad therapeutic potential. The purpose of the present work was to study the effect of alisporivir (2.5 mg/kg/day i.p.) on the ultrastructure and functions of the skeletal muscle mitochondria of mice with diabetes mellitus induced by a high-fat diet combined with streptozotocin injections. The glucose tolerance tests indicated that alisporivir increased the rate of glucose utilization in diabetic mice. An electron microscopy analysis showed that alisporivir prevented diabetes-induced changes in the ultrastructure and content of the mitochondria in myocytes. In diabetes, the ADP-stimulated respiration, respiratory control, and ADP/O ratios and the level of ATP synthase in the mitochondria decreased, whereas alisporivir treatment restored these indicators. Alisporivir eliminated diabetes-induced increases in mitochondrial lipid peroxidation products. Diabetic mice showed decreased mRNA levels of Atp5f1a, Ant1, and Ppif and increased levels of Ant2 in the skeletal muscles. The skeletal muscle mitochondria of diabetic animals were sensitized to the MPT pore opening. Alisporivir normalized the expression level of Ant2 and mitochondrial susceptibility to the MPT pore opening. In parallel, the levels of Mfn2 and Drp1 also returned to control values, suggesting a normalization of mitochondrial dynamics. These findings suggest that the targeting of the MPT pore opening by alisporivir is a therapeutic approach to prevent the development of mitochondrial dysfunction and associated oxidative stress in the skeletal muscles in diabetes.
Keywords: alisporivir; diabetes mellitus; lipid peroxidation; mitochondria; mitochondrial dysfunction; mitochondrial permeability transition pore.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Effect of the MPT Pore Inhibitor Alisporivir on the Development of Mitochondrial Dysfunction in the Heart Tissue of Diabetic Mice.Biology (Basel). 2021 Aug 28;10(9):839. doi: 10.3390/biology10090839. Biology (Basel). 2021. PMID: 34571715 Free PMC article.
-
Alisporivir Improves Mitochondrial Function in Skeletal Muscle of mdx Mice but Suppresses Mitochondrial Dynamics and Biogenesis.Int J Mol Sci. 2021 Sep 10;22(18):9780. doi: 10.3390/ijms22189780. Int J Mol Sci. 2021. PMID: 34575944 Free PMC article.
-
Chronic treatment with dapagliflozin protects against mitochondrial dysfunction in the liver of C57BL/6NCrl mice with high-fat diet/streptozotocin-induced diabetes mellitus.Mitochondrion. 2021 Jul;59:246-254. doi: 10.1016/j.mito.2021.06.008. Epub 2021 Jun 16. Mitochondrion. 2021. PMID: 34144205
-
Mitochondrial Cyclosporine A-Independent Palmitate/Ca2+-Induced Permeability Transition Pore (PA-mPT Pore) and Its Role in Mitochondrial Function and Protection against Calcium Overload and Glutamate Toxicity.Cells. 2021 Jan 11;10(1):125. doi: 10.3390/cells10010125. Cells. 2021. PMID: 33440765 Free PMC article. Review.
-
Mitochondrial permeability transition in CNS trauma: cause or effect of neuronal cell death?J Neurosci Res. 2005 Jan 1-15;79(1-2):231-9. doi: 10.1002/jnr.20292. J Neurosci Res. 2005. PMID: 15573402 Review.
Cited by
-
Modeling drug-induced mitochondrial toxicity with human primary cardiomyocytes.Sci China Life Sci. 2024 Feb;67(2):301-319. doi: 10.1007/s11427-023-2369-3. Epub 2023 Oct 18. Sci China Life Sci. 2024. PMID: 37864082
-
Mitophagy and mitochondrial dynamics in type 2 diabetes mellitus treatment.Aging (Albany NY). 2022 Mar 24;14(6):2902-2919. doi: 10.18632/aging.203969. Epub 2022 Mar 24. Aging (Albany NY). 2022. PMID: 35332108 Free PMC article. Review.
-
Targeting mitochondrial quality control: new therapeutic strategies for major diseases.Mil Med Res. 2024 Aug 21;11(1):59. doi: 10.1186/s40779-024-00556-1. Mil Med Res. 2024. PMID: 39164792 Free PMC article. Review.
-
Alisporivir Normalizes Mitochondrial Function of Primary Mouse Lung Endothelial Cells Under Conditions of Hyperglycemia.Biochemistry (Mosc). 2022 Jul;87(7):605-616. doi: 10.1134/S0006297922070033. Biochemistry (Mosc). 2022. PMID: 36154883 Free PMC article.
-
Pharmacological and Genetic Suppression of VDAC1 Alleviates the Development of Mitochondrial Dysfunction in Endothelial and Fibroblast Cell Cultures upon Hyperglycemic Conditions.Antioxidants (Basel). 2023 Jul 20;12(7):1459. doi: 10.3390/antiox12071459. Antioxidants (Basel). 2023. PMID: 37507997 Free PMC article.
References
-
- Patti M.E., Butte A.J., Crunkhorn S., Cusi K., Berria R., Kashyap S., Miyazaki Y., Kohane I., Costello M., Saccone R., et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc. Natl. Acad. Sci. USA. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

