Oligomeric and Fibrillar Species of Aβ42 Diversely Affect Human Neural Stem Cells
- PMID: 34502444
- PMCID: PMC8430695
- DOI: 10.3390/ijms22179537
Oligomeric and Fibrillar Species of Aβ42 Diversely Affect Human Neural Stem Cells
Abstract
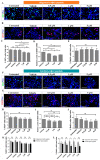
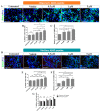
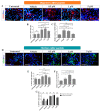
Amyloid-β 42 peptide (Aβ1-42 (Aβ42)) is well-known for its involvement in the development of Alzheimer's disease (AD). Aβ42 accumulates and aggregates in fibers that precipitate in the form of plaques in the brain causing toxicity; however, like other forms of Aβ peptide, the role of these peptides remains unclear. Here we analyze and compare the effects of oligomeric and fibrillary Aβ42 peptide on the biology (cell death, proliferative rate, and cell fate specification) of differentiating human neural stem cells (hNS1 cell line). By using the hNS1 cells we found that, at high concentrations, oligomeric and fibrillary Aβ42 peptides provoke apoptotic cellular death and damage of DNA in these cells, but Aβ42 fibrils have the strongest effect. The data also show that both oligomeric and fibrillar Aβ42 peptides decrease cellular proliferation but Aβ42 oligomers have the greatest effect. Finally, both, oligomers and fibrils favor gliogenesis and neurogenesis in hNS1 cells, although, in this case, the effect is more prominent in oligomers. All together the findings of this study may contribute to a better understanding of the molecular mechanisms involved in the pathology of AD and to the development of human neural stem cell-based therapies for AD treatment.
Keywords: Alzheimer’s; Aβ peptide; Aβ42; cell death; cell differentiation; fibrils; human NSCs; oligomers.
Conflict of interest statement
The authors confirm that this article content has no conflict of interest.
Figures



Similar articles
-
Aβ42 Peptide Promotes Proliferation and Gliogenesis in Human Neural Stem Cells.Mol Neurobiol. 2019 Jun;56(6):4023-4036. doi: 10.1007/s12035-018-1355-7. Epub 2018 Sep 27. Mol Neurobiol. 2019. PMID: 30259399
-
Effects of Amyloid-β Peptide on the Biology of Human Neural Stem Cells.Methods Mol Biol. 2018;1779:381-398. doi: 10.1007/978-1-4939-7816-8_23. Methods Mol Biol. 2018. PMID: 29886545
-
Neurogenesis Is Increased in Human Neural Stem Cells by Aβ40 Peptide.Int J Mol Sci. 2022 May 22;23(10):5820. doi: 10.3390/ijms23105820. Int J Mol Sci. 2022. PMID: 35628629 Free PMC article.
-
Conformational Essentials Responsible for Neurotoxicity of Aβ42 Aggregates Revealed by Antibodies against Oligomeric Aβ42.Molecules. 2022 Oct 10;27(19):6751. doi: 10.3390/molecules27196751. Molecules. 2022. PMID: 36235284 Free PMC article. Review.
-
Elucidating the Structures of Amyloid Oligomers with Macrocyclic β-Hairpin Peptides: Insights into Alzheimer's Disease and Other Amyloid Diseases.Acc Chem Res. 2018 Mar 20;51(3):706-718. doi: 10.1021/acs.accounts.7b00554. Epub 2018 Mar 6. Acc Chem Res. 2018. PMID: 29508987 Free PMC article. Review.
Cited by
-
Correlation of brain tissue volume loss with inflammatory biomarkers IL1β, P-tau, T-tau, and NLPR3 in the aging cognitively impaired population.Front Aging Neurosci. 2024 Jul 23;16:1388654. doi: 10.3389/fnagi.2024.1388654. eCollection 2024. Front Aging Neurosci. 2024. PMID: 39109268 Free PMC article.
-
Amyloid Precursor Protein (APP) Regulates Gliogenesis and Neurogenesis of Human Neural Stem Cells by Several Signaling Pathways.Int J Mol Sci. 2023 Aug 19;24(16):12964. doi: 10.3390/ijms241612964. Int J Mol Sci. 2023. PMID: 37629148 Free PMC article.
-
CHI3L1/YKL-40 signaling inhibits neurogenesis in models of Alzheimer's disease.Sci Adv. 2025 Jul 18;11(29):eadv1492. doi: 10.1126/sciadv.adv1492. Epub 2025 Jul 18. Sci Adv. 2025. PMID: 40680137 Free PMC article.
-
Natural antioxidants that act against Alzheimer's disease through modulation of the NRF2 pathway: a focus on their molecular mechanisms of action.Front Endocrinol (Lausanne). 2023 Jul 3;14:1217730. doi: 10.3389/fendo.2023.1217730. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37465125 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

