Cilia, Centrosomes and Skeletal Muscle
- PMID: 34502512
- PMCID: PMC8431768
- DOI: 10.3390/ijms22179605
Cilia, Centrosomes and Skeletal Muscle
Abstract
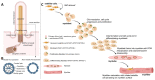
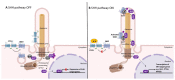
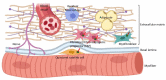
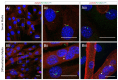
Primary cilia are non-motile, cell cycle-associated organelles that can be found on most vertebrate cell types. Comprised of microtubule bundles organised into an axoneme and anchored by a mature centriole or basal body, primary cilia are dynamic signalling platforms that are intimately involved in cellular responses to their extracellular milieu. Defects in ciliogenesis or dysfunction in cilia signalling underlie a host of developmental disorders collectively referred to as ciliopathies, reinforcing important roles for cilia in human health. Whilst primary cilia have long been recognised to be present in striated muscle, their role in muscle is not well understood. However, recent studies indicate important contributions, particularly in skeletal muscle, that have to date remained underappreciated. Here, we explore recent revelations that the sensory and signalling functions of cilia on muscle progenitors regulate cell cycle progression, trigger differentiation and maintain a commitment to myogenesis. Cilia disassembly is initiated during myoblast fusion. However, the remnants of primary cilia persist in multi-nucleated myotubes, and we discuss their potential role in late-stage differentiation and myofiber formation. Reciprocal interactions between cilia and the extracellular matrix (ECM) microenvironment described for other tissues may also inform on parallel interactions in skeletal muscle. We also discuss emerging evidence that cilia on fibroblasts/fibro-adipogenic progenitors and myofibroblasts may influence cell fate in both a cell autonomous and non-autonomous manner with critical consequences for skeletal muscle ageing and repair in response to injury and disease. This review addresses the enigmatic but emerging role of primary cilia in satellite cells in myoblasts and myofibers during myogenesis, as well as the wider tissue microenvironment required for skeletal muscle formation and homeostasis.
Keywords: cytoskeleton; differentiation; extracellular matrix; myogenesis; primary cilia; proliferation; satellite cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The role of primary cilia in myoblast proliferation and cell cycle regulation during myogenesis.Cell Struct Funct. 2025 Feb 18;50(1):53-63. doi: 10.1247/csf.24067. Epub 2025 Jan 10. Cell Struct Funct. 2025. PMID: 39805615 Review.
-
A novel in vitro model for the assessment of postnatal myonuclear accretion.Skelet Muscle. 2018 Feb 14;8(1):4. doi: 10.1186/s13395-018-0151-4. Skelet Muscle. 2018. PMID: 29444710 Free PMC article.
-
Regulation of IRS1/Akt insulin signaling by microRNA-128a during myogenesis.J Cell Sci. 2013 Jun 15;126(Pt 12):2678-91. doi: 10.1242/jcs.119966. Epub 2013 Apr 19. J Cell Sci. 2013. PMID: 23606743 Free PMC article.
-
The Centrosome and the Primary Cilium: The Yin and Yang of a Hybrid Organelle.Cells. 2019 Jul 10;8(7):701. doi: 10.3390/cells8070701. Cells. 2019. PMID: 31295970 Free PMC article. Review.
-
Platelet releasate promotes skeletal myogenesis by increasing muscle stem cell commitment to differentiation and accelerates muscle regeneration following acute injury.Acta Physiol (Oxf). 2019 Mar;225(3):e13207. doi: 10.1111/apha.13207. Epub 2018 Nov 14. Acta Physiol (Oxf). 2019. PMID: 30339324
Cited by
-
Hallmarks of ageing in human skeletal muscle and implications for understanding the pathophysiology of sarcopenia in women and men.Clin Sci (Lond). 2023 Nov 29;137(22):1721-1751. doi: 10.1042/CS20230319. Clin Sci (Lond). 2023. PMID: 37986616 Free PMC article.
-
CCDC78: Unveiling the Function of a Novel Gene Associated with Hereditary Myopathy.Cells. 2024 Sep 8;13(17):1504. doi: 10.3390/cells13171504. Cells. 2024. PMID: 39273074 Free PMC article.
-
Structure, function, and research progress of primary cilia in reproductive physiology and reproductive diseases.Front Cell Dev Biol. 2024 Jun 3;12:1418928. doi: 10.3389/fcell.2024.1418928. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38887518 Free PMC article. Review.
-
Absence of the primary cilia formation gene Talpid3 impairs muscle stem cell function.Commun Biol. 2023 Nov 4;6(1):1121. doi: 10.1038/s42003-023-05503-9. Commun Biol. 2023. PMID: 37925530 Free PMC article.
-
The Role of Integrin β1D Mislocalization in the Pathophysiology of Calpain 3-Related Limb-Girdle Muscular Dystrophy.Cells. 2025 Mar 17;14(6):446. doi: 10.3390/cells14060446. Cells. 2025. PMID: 40136695 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

