Methotrexate Ameliorates Systemic Inflammation and Septic Associated-Lung Damage in a Cecal Ligation and Puncture Septic Rat Model
- PMID: 34502521
- PMCID: PMC8431751
- DOI: 10.3390/ijms22179612
Methotrexate Ameliorates Systemic Inflammation and Septic Associated-Lung Damage in a Cecal Ligation and Puncture Septic Rat Model
Abstract
Background: Sepsis is a serious, heterogeneous clinical entity produced by a severe and systemic host inflammatory response to infection. Methotrexate (MTX) is a folate-antagonist that induces the generation of adenosine and also inhibits JAK/STAT pathway; MTX it is widely used as an anti-inflammatory drug to control the immune system.
Objective: The aim of this study was to assess the beneficial effects of a single and low dose of MTX in the systemic response and acute lung injury (ALI) induced by sepsis. As in the clinics, we treated our animals with antibiotics and fluids and performed the source control to mimic the current clinic treatment.
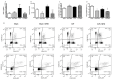
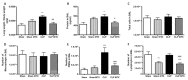
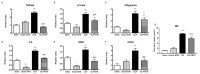
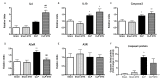
Methods and main results: Sepsis was induced in rats by a cecal ligation puncture (CLP) procedure. Six hours after induction of sepsis, we proceeded to the source control; fluids and antibiotics were administered at 6 h and 24 h after CLP. MTX (2.5 mg/Kg) was administered 6 h after the first surgery in one CLP experimental group and to one Sham group. A protective effect of MTX was observed through a significant reduction of pro-inflammatory cytokines and a decrease infiltration of inflammatory cells in the lung. In addition, we found a regulation in adenosine receptor A2aR and the metalloproteinases by MTX.
Conclusion: A single, low dose of MTX attenuates sepsis lung-associated damage by decreasing pro-inflammatory response, infiltration of pro-inflammatory cells and avoiding defective tissue lung remodeling.
Keywords: acute lung injury; acute respiratory distress syndrome; methotrexate; sepsis; systemic inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Senegenin Ameliorate Acute Lung Injury Through Reduction of Oxidative Stress and Inhibition of Inflammation in Cecal Ligation and Puncture-Induced Sepsis Rats.Inflammation. 2016 Apr;39(2):900-6. doi: 10.1007/s10753-016-0322-6. Inflammation. 2016. PMID: 26945584
-
A newly synthetic vitamin E derivative, E-Ant-S-GS, attenuates lung injury caused by cecal ligation and puncture-induced sepsis in rats.Surgery. 2012 Mar;151(3):420-6. doi: 10.1016/j.surg.2011.08.003. Epub 2011 Oct 13. Surgery. 2012. PMID: 22000829
-
(R)-Ketamine ameliorates lethal inflammatory responses and multi-organ injury in mice induced by cecum ligation and puncture.Life Sci. 2021 Nov 1;284:119882. doi: 10.1016/j.lfs.2021.119882. Epub 2021 Aug 10. Life Sci. 2021. PMID: 34384829
-
Hydrogen-rich saline ameliorates lung injury associated with cecal ligation and puncture-induced sepsis in rats.Exp Mol Pathol. 2015 Apr;98(2):268-76. doi: 10.1016/j.yexmp.2015.03.005. Epub 2015 Mar 4. Exp Mol Pathol. 2015. PMID: 25746665 Review.
-
Glucans: A Therapeutic Alternative for Sepsis Treatment.J Immunol Res. 2024 May 27;2024:6876247. doi: 10.1155/2024/6876247. eCollection 2024. J Immunol Res. 2024. PMID: 38939744 Free PMC article. Review.
Cited by
-
Signaling pathways and potential therapeutic targets in acute respiratory distress syndrome (ARDS).Respir Res. 2024 Jan 13;25(1):30. doi: 10.1186/s12931-024-02678-5. Respir Res. 2024. PMID: 38218783 Free PMC article. Review.
-
INDUCTION OF EARLY PULMONARY SENESCENCE IN EXPERIMENTAL SEPSIS.Shock. 2025 Mar 1;63(3):448-455. doi: 10.1097/SHK.0000000000002512. Epub 2024 Dec 4. Shock. 2025. PMID: 39637172 Free PMC article.
-
Acute respiratory distress syndrome heterogeneity and the septic ARDS subgroup.Front Immunol. 2023 Nov 14;14:1277161. doi: 10.3389/fimmu.2023.1277161. eCollection 2023. Front Immunol. 2023. PMID: 38035100 Free PMC article. Review.
-
Protective Effects and Mechanisms of Luteolin against Acute Respiratory Distress Syndrome: Network Pharmacology and In vivo and In vitro Studies.Curr Pharm Des. 2024;30(18):1404-1418. doi: 10.2174/0113816128289341240327072531. Curr Pharm Des. 2024. PMID: 38616753
-
Phospholipid transfer protein ameliorates sepsis-induced cardiac dysfunction through NLRP3 inflammasome inhibition.Open Med (Wars). 2024 Mar 27;19(1):20240915. doi: 10.1515/med-2024-0915. eCollection 2024. Open Med (Wars). 2024. PMID: 38584827 Free PMC article.
References
-
- Dellinger R.P., Levy M.M., Rhodes A., Annane D., Gerlach H., Opal S.M., Sevransky J.E., Sprung C.L., Douglas I.S., Jaeschke R., et al. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock: 2012. Crit. Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. - DOI - PubMed
-
- Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.-D., Coopersmith C.M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. - DOI - PMC - PubMed
-
- Martínez M.L., Ferrer R., Torrents E., Guillamat-Prats R., Gomà G., Suárez D., Álvarez-Rocha L., Pozo Laderas J.C., Martín-Loeches I., Levy M.M., et al. Impact of Source Control in Patients With Severe Sepsis and Septic Shock. Crit. Care Med. 2017;45:11–19. doi: 10.1097/CCM.0000000000002011. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

