Multiphoton Laser Fabrication of Hybrid Photo-Activable Biomaterials
- PMID: 34502787
- PMCID: PMC8433654
- DOI: 10.3390/s21175891
Multiphoton Laser Fabrication of Hybrid Photo-Activable Biomaterials
Abstract
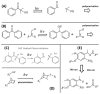
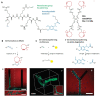
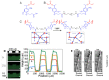
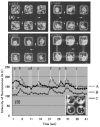
The possibility to shape stimulus-responsive optical polymers, especially hydrogels, by means of laser 3D printing and ablation is fostering a new concept of "smart" micro-devices that can be used for imaging, thermal stimulation, energy transducing and sensing. The composition of these polymeric blends is an essential parameter to tune their properties as actuators and/or sensing platforms and to determine the elasto-mechanical characteristics of the printed hydrogel. In light of the increasing demand for micro-devices for nanomedicine and personalized medicine, interest is growing in the combination of composite and hybrid photo-responsive materials and digital micro-/nano-manufacturing. Existing works have exploited multiphoton laser photo-polymerization to obtain fine 3D microstructures in hydrogels in an additive manufacturing approach or exploited laser ablation of preformed hydrogels to carve 3D cavities. Less often, the two approaches have been combined and active nanomaterials have been embedded in the microstructures. The aim of this review is to give a short overview of the most recent and prominent results in the field of multiphoton laser direct writing of biocompatible hydrogels that embed active nanomaterials not interfering with the writing process and endowing the biocompatible microstructures with physically or chemically activable features such as photothermal activity, chemical swelling and chemical sensing.
Keywords: 3D printing; hydrogels; photo-ablation; photo-polymerization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


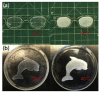
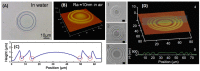


References
-
- Chirico G., Dacarro G., O’Regan C., Peltonen J., Sarfraz J., Taglietti A., Borzenkov M., Pallavicini P. Photothermally responsive inks for inkjet-printing secure information. Part. Part. Syst. Charact. 2018;5:1800095. doi: 10.1002/ppsc.201800095. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

