Application of β-Tricalcium Phosphate in Adhesive Dentin Bonding
- PMID: 34502894
- PMCID: PMC8434446
- DOI: 10.3390/polym13172855
Application of β-Tricalcium Phosphate in Adhesive Dentin Bonding
Abstract
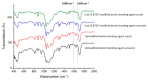
The study aimed at synthesizing β-tricalcium phosphate (β-TCP) nanoparticles and comparing the mechanical properties and dentin interaction of two adhesives: experimental adhesive (EA) and EA with 5 wt.% β-TCP nanoparticles (β-TCP-5%). These filler nanoparticles were synthesized and then characterized with scanning electron microscopy (SEM) and micro-Raman spectroscopy. The β-TCP nanoparticles were incorporated in the adhesives to form two groups: gp-1: EA (control) and gp-2: β-TCP-5%. These adhesives were characterized by SEM, energy-dispersive X-ray (EDX) spectroscopy and were also assessed for their micro-tensile bond strength (μTBS) with (TC) and without thermocycling (NTC). Fourier Transform Infrared (FTIR) spectroscopy was performed to evaluate the degree of conversion (DC) of two adhesives. The β-TCP filler was seen as irregularly shaped agglomerates on SEM. The micro-Raman spectra revealed characteristic peaks associated with β-TCP nanoparticles. Both adhesives presented suitable dentin interaction, which was demonstrated by the formation of resin tags of variable depths. The EDX analysis verified the existence of calcium (Ca) and phosphate (P) for the β-TCP-5% group. The greatest μTBS values were shown by β-TCP-5% group samples when they were non-thermocycled (NTC) (β-TCP-5%-NTC: 34.11 ± 3.46) followed by the thermocycled (TC) samples of the same group (β-TCP-5%-TC: 30.38 ± 3.66), compared with the EA group. Although the DC presented by β-TCP-5% group was comparable to the EA group, it was still lower. The addition of β-TCP nanoparticles in the adhesive improved its μTBS and resulted in a suitable dentin interaction, seen in the form of hybrid layer and resin tag formation. Nonetheless, a decreased DC was observed for the β-TCP-5% adhesive. Future studies probing the effect of different filler concentrations on various properties of the adhesive are warranted.
Keywords: adhesive; calcium; dentin; phosphate; tricalcium phosphate.
Conflict of interest statement
None declared.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

