Thermal Ablation versus Stereotactic Ablative Body Radiotherapy to Treat Unresectable Colorectal Liver Metastases: A Comparative Analysis from the Prospective Amsterdam CORE Registry
- PMID: 34503113
- PMCID: PMC8428373
- DOI: 10.3390/cancers13174303
Thermal Ablation versus Stereotactic Ablative Body Radiotherapy to Treat Unresectable Colorectal Liver Metastases: A Comparative Analysis from the Prospective Amsterdam CORE Registry
Abstract
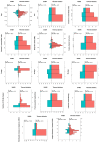
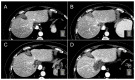
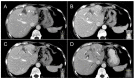
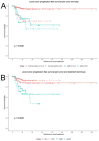
Thermal ablation and stereotactic ablative radiotherapy (SABR) are techniques to eradicate colorectal liver metastases (CRLM). This study compares the safety, efficacy and long-term oncological outcomes of these treatment methods. All prospectively registered patients (AmCORE registry) treated with thermal ablation or SABR alone for unresectable CRLM between 2007 and 2020 were analyzed using multivariate Cox-proportional hazard regression. In total 199 patients were included for analysis: 144 (400 CRLM) thermal ablation; 55 (69 CRLM) SABR. SABR patients were characterized by older age (p = 0.006), extrahepatic disease at diagnosis (p = 0.004) and larger tumors (p < 0.001). Thermal ablation patients were more likely to have synchronous disease, higher clinical risk scores (p = 0.030) and higher numbers of CRLMs treated (p < 0.001). Mortality was zero and morbidity low in both groups: no serious adverse events were recorded following SABR (n = 0/55) and nine (n = 9/144 [6.3%]; all CTCAE grade 3) after thermal ablation. SABR was associated with an inferior overall survival (OS) (median OS 53.0 months vs. 27.4 months; HR = 1.29, 95% CI 1.12-1.49; p = 0.003), local tumor progression-free survival (LTPFS) per-tumor (HR = 1.24, 95% CI 1.01-1.52; p = 0.044) and local control per-patient (HR = 1.57, 95% CI 1.20-2.04; p = 0.001) and per-tumor (HR = 1.89, 95% CI 1.44-2.49; p < 0.001). In this study thermal ablation was superior to SABR with regard to OS, LTPFS and local control, albeit at the cost of a limited risk of serious adverse events. Further studies are required to assess whether the worse outcomes following SABR were the effect of true differences in ablative treatment or a result of residual confounding.
Keywords: colorectal liver metastases (CRLM); microwave ablation (MWA); radiofrequency ablation (RFA); stereotactic ablative radiotherapy (SABR); thermal ablation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- WHO Estimated Age-Standardized Incidence Rates (World) in 2018, All Cancers, Both Sexes, All Ages. [(accessed on 1 April 2021)];2018 Available online: http://gco.iarc.fr/today/online-analysis-map.
LinkOut - more resources
Full Text Sources

