Centralised RECIST Assessment and Clinical Outcomes with Lenvatinib Monotherapy in Recurrent and Metastatic Adenoid Cystic Carcinoma
- PMID: 34503145
- PMCID: PMC8431195
- DOI: 10.3390/cancers13174336
Centralised RECIST Assessment and Clinical Outcomes with Lenvatinib Monotherapy in Recurrent and Metastatic Adenoid Cystic Carcinoma
Abstract
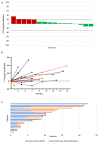
Adenoid cystic carcinoma (ACC) is a rare cancer of secretory glands. Recurrent or metastatic (R/M) ACC is generally considered resistant to cytotoxic chemotherapy. Recent phase II studies have reported improved objective response rates (ORR) with the use of the multi-kinase inhibitor lenvatinib. We sought to evaluate real-world experience of R/M ACC patients treated with lenvatinib monotherapy within the UK National Health Service (NHS) to determine the response rates by Response Evaluation Criteria of Solid Tumour (RECIST) and clinical outcomes. Twenty-three R/M ACC patients from eleven cancer centres were included. All treatment assessments for clinical decision making related to drug therapy were undertaken at the local oncology centre. Central radiology review was performed by an independent clinical trial radiologist and blinded to the clinical decision making. In contrast to previously reported ORR of 12-15%, complete or partial response was not observed in any patients. Eleven patients (52.4%) had stable disease and 5 patients (23.8%) had progression of disease as the best overall response. The median time on treatment was 4 months and the median survival from discontinuation was 1 month. The median PFS and OS from treatment initiation were 4.5 months and 12 months respectively. Multicentre collaborative studies such as this are required to evaluate rare cancers with no recommended standard of care therapy and variable disease courses.
Keywords: adenoid cystic carcinoma; lenvatinib; salivary gland cancer.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. Additional financial disclosures are as follows: OD–Sanofi, Leo Pharma, Achilles Therapeutics; RM (Rafael Moleron)–MSD, BMS; PS–Abbvie, Philips, Accuray.
Figures



References
-
- Ellington C.L., Goodman M., Kono S.A., Grist W., Wadsworth T., Chen A.Y., Owonikoko T., Ramalingam S., Shin D.M., Khuri F.R., et al. Adenoid cystic carcinoma of the head and neck: Incidence and survival trends based on 1973–2007 Surveillance, Epidemiology, and End Results data. Cancer. 2012;118:4444–4451. doi: 10.1002/cncr.27408. - DOI - PubMed
LinkOut - more resources
Full Text Sources

