Apoptosis Deregulation and the Development of Cancer Multi-Drug Resistance
- PMID: 34503172
- PMCID: PMC8430856
- DOI: 10.3390/cancers13174363
Apoptosis Deregulation and the Development of Cancer Multi-Drug Resistance
Abstract
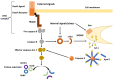
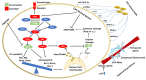
The ability of tumor cells to evade apoptosis is established as one of the hallmarks of cancer. The deregulation of apoptotic pathways conveys a survival advantage enabling cancer cells to develop multi-drug resistance (MDR), a complex tumor phenotype referring to concurrent resistance toward agents with different function and/or structure. Proteins implicated in the intrinsic pathway of apoptosis, including the Bcl-2 superfamily and Inhibitors of Apoptosis (IAP) family members, as well as their regulator, tumor suppressor p53, have been implicated in the development of MDR in many cancer types. The PI3K/AKT pathway is pivotal in promoting survival and proliferation and is often overactive in MDR tumors. In addition, the tumor microenvironment, particularly factors secreted by cancer-associated fibroblasts, can inhibit apoptosis in cancer cells and reduce the effectiveness of different anti-cancer drugs. In this review, we describe the main alterations that occur in apoptosis-and related pathways to promote MDR. We also summarize the main therapeutic approaches against resistant tumors, including agents targeting Bcl-2 family members, small molecule inhibitors against IAPs or AKT and agents of natural origin that may be used as monotherapy or in combination with conventional therapeutics. Finally, we highlight the potential of therapeutic exploitation of epigenetic modifications to reverse the MDR phenotype.
Keywords: Bcl-2 family of proteins; PI3K/AKT pathway; apoptosis; cancer associated fibroblasts; cancer therapy; caspase-dependent death; epigenetic modifications; multi-drug resistance; tumor-microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Tsaur I., Heidegger I., Kretschmer A., Borgmann H., Gandaglia G., Briganti A., de Visschere P., Mathieu R., Valerio M., van den Bergh R., et al. Aggressive variants of prostate cancer—Are we ready to apply specific treatment right now? Cancer Treat. Rev. 2019;75:20–26. doi: 10.1016/j.ctrv.2019.03.001. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

