Health-Related Quality of Life in Oral Cancer Patients: Scoping Review and Critical Appraisal of Investigated Determinants
- PMID: 34503208
- PMCID: PMC8431462
- DOI: 10.3390/cancers13174398
Health-Related Quality of Life in Oral Cancer Patients: Scoping Review and Critical Appraisal of Investigated Determinants
Abstract
Background: health-related quality of life (HRQOL) represents a secondary endpoint of medical interventions in oncological patients. Our aim was to highlight potential sources of bias that could be encountered when evaluating HRQOL in oral cancer patients.
Methods: this review followed PRISMA-ScR recommendations.
Participants: patients treated for oral cancer.
Concept: HRQOL assessed by EORTC QLQ-C30 and QLQ-H&N35/QLQ-H&N43. A critical appraisal of included studies was performed to evaluate the accuracy of data stratification with respect to HRQOL determinants.
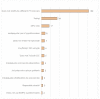
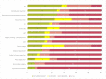
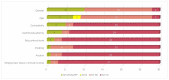
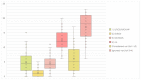
Results: overall, 30 studies met the inclusion criteria, totaling 1833 patients. In total, 8 sociodemographic (SDG) and 15 disease/treatment-specific (DT) HRQOL determinants (independent variables) were identified. The mean number of the independent variables was 6.1 (SD, 4.3)-5.0 (SD, 4.0) DT-related and 1.1 (SD, 1.8) SDG-related variables per article. None of the included papers considered all the identified determinants simultaneously.
Conclusions: a substantial lack of evidence regarding HRQOL determinants was demonstrated. This strongly weakens the reliability of the reported findings due to the challenging presence of baseline confounding, selection, and omitted variable biases. The proposed approach recommends the use of further evaluation tools that gather more variables in a single score together with a selection of more homogeneous, reproducible, and comparable cohorts based on the identified baseline confounding.
Keywords: HRQOL; head; neck; oncology; oral cancer; quality of life.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- European Medicines Agency. Pre-Authorisation Evaluation of Medicines for Human Use. Doc. Ref. EMEA/CHMP/EWP/139391/2004. London. [(accessed on 28 August 2021)];2005 Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-p....
-
- Apolone G., De Carli G., Brunetti M., Garattini S. Health-related quality of life (HR-QOL) and regulatory issues. An assessment of the European Agency for the Evaluation of Medicinal Products (EMEA) recommendations on the use of HR-QOL measures in drug approval. PharmacoEconomics. 2001;19:187–195. doi: 10.2165/00019053-200119020-00005. - DOI - PubMed
-
- Marquis P., Caron M., Emery M.-P., Scott J.A., Arnould B., Acquadro C. The Role of Health-Related Quality of Life Data in the Drug Approval Processes in the US and Europe. Pharm. Med. 2011;25:147–160. doi: 10.1007/BF03256856. - DOI
-
- Ferrans C.E.P. Definitions and Conceptual Models of Quality of Life. Outcomes Assessment in Cancer: Measures, Methods, and Applications. Cambridge University Press (CUP); New York, NY, USA: 2005. pp. 14–30.
-
- Heckscher A.L. Goals for Americans: (The Report of the Presidential Commission on National Goals and Chapters Submitted for the Consideration of the Commission of the American Assembly) Prentice-Hall Washington; Washington, DC, USA: 1960. The quality of American culture; pp. 127–146.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

