NK Cells in Myeloproliferative Neoplasms (MPN)
- PMID: 34503210
- PMCID: PMC8431564
- DOI: 10.3390/cancers13174400
NK Cells in Myeloproliferative Neoplasms (MPN)
Abstract
Myeloproliferative neoplasms (MPNs) comprise a heterogenous group of hematologic neoplasms which are divided into Philadelphia positive (Ph+), and Philadelphia negative (Ph-) or classical MPNs. A variety of immunological factors including inflammatory, as well as immunomodulatory processes, closely interact with the disease phenotypes in MPNs. NK cells are important innate immune effectors and substantially contribute to tumor control. Changes to the absolute and proportionate numbers of NK cell, as well as phenotypical and functional alterations are seen in MPNs. In addition to the disease itself, a variety of therapeutic options in MPNs may modify NK cell characteristics. Reports of suppressive effects of MPN treatment strategies on NK cell activity have led to intensive investigations into the respective compounds, to elucidate the possible negative effects of MPN therapy on control of the leukemic clones. We hereby review the available literature on NK cells in Ph+ and Ph- MPNs and summarize today's knowledge on disease-related alterations in this cell compartment with particular focus on known therapy-associated changes. Furthermore, we critically evaluate conflicting data with possible implications for future projects. We also aim to highlight the relevance of full NK cell functionality for disease control in MPNs and the importance of considering specific changes related to therapy in order to avoid suppressive effects on immune surveillance.
Keywords: CML; Innate immunity; essential thrombocythemia; polycythemia vera; primary myelofibrosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
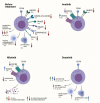
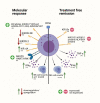
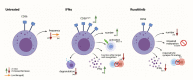
References
-
- Barbui T., Thiele J., Gisslinger H., Kvasnicka H.M., Vannucchi A.M., Guglielmelli P., Orazi A., Tefferi A. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: Document summary and in-depth discussion. Blood Cancer J. 2018;8:1–11. doi: 10.1038/s41408-018-0054-y. - DOI - PMC - PubMed
-
- Arber D.A., Orazi A., Hasserjian R., Thiele J., Borowitz M.J., Le Beau M.M., Bloomfield C.D., Cazzola M., Vardiman J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

