Large Granular Lymphocytic Leukemia: From Immunopathogenesis to Treatment of Refractory Disease
- PMID: 34503230
- PMCID: PMC8430581
- DOI: 10.3390/cancers13174418
Large Granular Lymphocytic Leukemia: From Immunopathogenesis to Treatment of Refractory Disease
Abstract
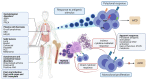
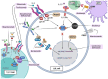
Large Granular Lymphocyte Leukemia (LGLL) is a rare, chronic lymphoproliferative disorder of effector cytotoxic T-cells, and less frequently, natural killer (NK) cells. The disease is characterized by an indolent and often asymptomatic course. However, in roughly 50% of cases, treatment is required due to severe transfusion-dependent anemia, severe neutropenia, or moderate neutropenia with associated recurrent infections. LGLL represents an interesting disease process at the intersection of a physiological immune response, autoimmune disorder, and malignant (clonal) proliferation, resulting from the aberrant activation of cellular pathways promoting survival, proliferation, and evasion of apoptotic signaling. LGLL treatment primarily consists of immunosuppressive agents (methotrexate, cyclosporine, and cyclophosphamide), with a cumulative response rate of about 60% based on longitudinal expertise and retrospective studies. However, refractory cases can result in clinical scenarios characterized by transfusion-dependent anemia and severe neutropenia, which warrant further exploration of other potential targeted treatment modalities. Here, we summarize the current understanding of the immune-genomic profiles of LGLL, its pathogenesis, and current treatment options, and discuss potential novel therapeutic agents, particularly for refractory disease.
Keywords: immunogenomics; large granular lymphocytic leukemia; refractory disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Zambello R., Loughran T.P.J., Trentin L., Pontisso P., Battistella L., Raimondi R., Facco M., Sancetta R., Agostini C., Pizzolo G. Serologic and molecular evidence for a possible pathogenetic role of viral infection in CD3-negative natural killer-type lymphoproliferative disease of granular lymphocytes. Leukemia. 1995;9:1207–1211. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

