Clinical Importance of Regimens in Hepatic Arterial Infusion Chemotherapy for Advanced Hepatocellular Carcinoma with Macrovascular Invasion
- PMID: 34503259
- PMCID: PMC8431395
- DOI: 10.3390/cancers13174450
Clinical Importance of Regimens in Hepatic Arterial Infusion Chemotherapy for Advanced Hepatocellular Carcinoma with Macrovascular Invasion
Abstract
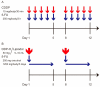
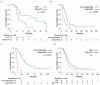
Macroscopic vascular invasion (MVI) is a poor prognostic factor in hepatocellular carcinoma (HCC). Hepatic arterial infusion chemotherapy (HAIC) is a promising treatment in MVI-HCC. However, it is not clear which regimens are suitable for HAIC. In this study, we aimed to compare the therapeutic effects between New FP (a fine-powder cisplatin suspended with lipiodol plus 5-fluorouracil) and low dose FP (LFP/cisplatin plus 5-fluorouracil) in the treatment of MVI-HCC patients with Child-Pugh class A. New FP is a regimen that consists of a fine-powder cisplatin suspended with lipiodol and 5-fluorouracil. Fifty-one patients were treated with LFP, and 99 patients were New FP. We compared the therapeutic effects of LFP and New FP and assessed factors that associated with the therapeutic effects. The median survival and progression-free survival times of LFP and New FP were 16.1/24.7 and 5.4/8.8 months, respectively (p < 0.05, p < 0.05). The complete response (29%) and objective response rate (76%) of New FP were significantly higher than those of LFP (p < 0.001, p < 0.01). Factors associated with better therapeutic response were better ALBI-grade and New FP treatment choice. New FP is a more powerful regimen than LFP in HAIC for MVI-HCC. New FP represents a recommended HAIC regimen for the treatment of patients with MVI-HCC.
Keywords: New FP; hepatic arterial infusion chemotherapy; hepatocellular carcinoma; low dose FP; macrovascular invasion; vascular invasion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kudo M., Izumi N., Kokudo N., Matsui O., Sakamoto M., Nakashima O., Kojiro M., Makuuchi M. Management of Hepatocellular Carcinoma in Japan: Consensus-Based Clinical Practice Guidelines Proposed by the Japan Society of Hepatology (JSH) 2010 Updated Version. Dig. Dis. 2011;29:339–364. doi: 10.1159/000327577. - DOI - PubMed
LinkOut - more resources
Full Text Sources

