Effects of microglial depletion and TREM2 deficiency on Aβ plaque burden and neuritic plaque tau pathology in 5XFAD mice
- PMID: 34503586
- PMCID: PMC8428059
- DOI: 10.1186/s40478-021-01251-1
Effects of microglial depletion and TREM2 deficiency on Aβ plaque burden and neuritic plaque tau pathology in 5XFAD mice
Abstract
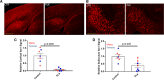
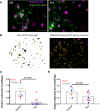
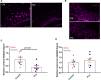
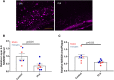
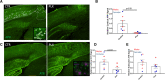
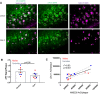
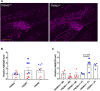
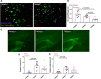
Dystrophic neuronal processes harboring neuritic plaque (NP) tau pathology are found in association with Aβ plaques in Alzheimer's disease (AD) brain. Microglia are also in proximity to these plaques and microglial gene variants are known risk factors in AD, including loss-of-function variants of TREM2. We have further investigated the role of Aβ plaque-associated microglia in 5XFAD mice in which NP tau pathology forms after intracerebral injection of AD brain-derived pathologic tau (AD-tau), focusing on the consequences of reduced TREM2 expression and microglial depletion after treatment with the colony-stimulating factor 1 (CSFR1) inhibitor, PLX3397. Young 5XFAD mice treated with PLX3397 had a large reduction of brain microglia, including cortical plaque-associated microglia, with a significant reduction of Aβ plaque burden in the cortex. A corresponding decrease in cortical APP-positive dystrophic processes and NP tau pathology were observed after intracerebral AD-tau injection in the PLX3397-treated 5XFAD mice. Consistent with prior reports, 5XFAD × TREM2-/- mice showed a significant reduction of plaque-associated microglial, whereas 5XFAD × TREM2+/- mice had significantly more plaque-associated microglia than 5XFAD × TREM2-/- mice. Nonetheless, AD-tau injected 5XFAD × TREM2+/- mice showed greatly increased AT8-positive NP tau relative to 5XFAD × TREM2+/+ mice. Expression profiling revealed that 5XFAD × TREM2+/- mice had a disease-associated microglial (DAM) gene expression profile in the brain that was generally intermediate between 5XFAD × TREM2+/+ and 5XFAD × TREM2-/- mice. Microarray analysis revealed significant differences in cortical and hippocampal gene expression between AD-tau injected 5XFAD × TREM2+/- and 5XFAD × TREM2-/- mice, including pathways linked to microglial function. These data suggest there is not a simple correlation between the extent of microglia plaque interaction and plaque-associated neuritic damage. Moreover, the differences in gene expression and microglial phenotype between TREM2+/- and TREM2-/- mice suggest that the former may better model the single copy TREM2 variants associated with AD risk.
Keywords: Alzheimer’s; Microglia; Pathology; Plaques; Tau.
© 2021. The Author(s).
Conflict of interest statement
Certain of the authors are employees of Janssen Pharmaceutica, NV, who provided funding for these studies.
Figures

References
-
- Atagi Y, Liu CC, Painter MM, Chen XF, Verbeeck C, Zheng H, Li, Rademakers R, Kang SS, Xu H, Younkin S, Das P, Fryer JD, Bu G. Apolipoprotein E is a ligand for triggering receptor expressed on myeloid cells 2 (TREM2) J Biol Chem. 2015;290:26043–26050. doi: 10.1074/jbc.M115.679043. - DOI - PMC - PubMed
-
- Ball M, Braak H, Coleman P, Dickson D, Duyckaerts C, Gambetti P, Hansen L, Hyman B, Jellinger K, Markesbery W, Perl D, Powers J, Price J, Trojanowski JQ, Wisniewski H, Phelps C, Khachaturian Z. Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. Neurobiol Aging. 1997;18:S1–S2. doi: 10.1016/S0197-4580(97)00052-3. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

