Medial Arterial Calcification: JACC State-of-the-Art Review
- PMID: 34503684
- PMCID: PMC8439554
- DOI: 10.1016/j.jacc.2021.06.049
Medial Arterial Calcification: JACC State-of-the-Art Review
Abstract
Medial arterial calcification (MAC) is a chronic systemic vascular disorder distinct from atherosclerosis that is frequently but not always associated with diabetes mellitus, chronic kidney disease, and aging. MAC is also a part of more complex phenotypes in numerous less common diseases. The hallmarks of MAC include disseminated and progressive precipitation of calcium phosphate within the medial layer, a prolonged and clinically silent course, and compromise of hemodynamics associated with chronic limb-threatening ischemia. MAC increases the risk of complications during vascular interventions and mitigates their outcomes. With the exception of rare monogenetic defects affecting adenosine triphosphate metabolism, MAC pathogenesis remains unknown, and causal therapy is not available. Implementation of genetics and omics-based approaches in research recognizing the critical importance of calcium phosphate thermodynamics holds promise to unravel MAC molecular pathogenesis and to provide guidance for therapy. The current state of knowledge concerning MAC is reviewed, and future perspectives are outlined.
Keywords: atherosclerosis; chronic limb-threatening ischemia; genetics; medial arterial calcification; omics; peripheral artery disease; vascular calcification.
Copyright © 2021 American College of Cardiology Foundation. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures Dr Furniss is supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre. Dr Schuchardt has received support from Charité 3R and the Bundesministerium für Bildung und Forschung. Dr J.D. Lanzer has received support from Informatics for Life funded by the Klaus Tschira Foundation. Dr Thakker has received support from a Wellcome Trust Senior Investigator Award, an NIHR Senior Investigator Award, and the NIHR Oxford Biomedical Research Centre Programme. Dr Saez-Rodriguez has received support from Informatics for Life funded by the Klaus Tschira Foundation; has received funding from GSK and Sanofi; and expects consultant fees from Travere Therapeutics. Dr Millan has received financial support from the Spanish Ministry of Science, Innovation, and Universities (grant no: PGC2018_095795_B_I00). Dr Sato has received institutional research support from NIH-HL141425, Leducq Foundation Grant, 480 Biomedical, 4C Medical, 4Tech, Abbott, Accumedical, Alivas, Amgen, Biosensors, Boston Scientific, Canon USA, Cardiac Implants, Celonova, Claret Medical, Concept Medical, Cook, CSI, DuNing, Inc, Edwards LifeSciences, Emboline, Endotronix, Envision Scientific, Lutonix/Bard, Gateway, Lifetech, Limflo, MedAlliance, Medtronic, Mercator, Merill, Microport Medical, Microvention, Mitraalign, Mitra assist, NAMSA, Nanova, Neovasc, NIPRO, Novogate, Occulotech, OrbusNeich Medical, Phenox, Profusa, Protembis, Qool, ReCor Medical, Senseonics, Shockwave, Sinomed, Spectranetics, Surmodics, Symic, Vesper, W.L. Gore, and Xeltis. Dr Virmani has received institutional research support from NIH-HL141425, Leducq Foundation Grant, 480 Biomedical, 4C Medical, 4Tech, Abbott, Accumedical, Alivas, Amgen, Biosensors, Boston Scientific, Canon USA, Cardiac Implants, Celonova, Claret Medical, Concept Medical, Cook, CSI, DuNing, Inc, Edwards LifeSciences, Emboline, Endotronix, Envision Scientific, Lutonix/Bard, Gateway, Lifetech, Limflo, MedAlliance, Medtronic, Mercator, Merill, Microport Medical, Microvention, Mitraalign, Mitra assist, NAMSA, Nanova, Neovasc, NIPRO, Novogate, Occulotech, OrbusNeich Medical, Phenox, Profusa, Protembis, Qool, ReCor Medical, Senseonics, Shockwave, Sinomed, Spectranetics, Surmodics, Symic, Vesper, W.L. Gore, and Xeltis; is a consultant for Abbott Vascular, Boston Scientific, Celonova, OrbusNeich Medical, Terumo Corporation, W.L. Gore, Edwards Lifesciences, Cook Medical, CSI, ReCor Medical, SinoMedical Sciences Technology, Surmodics, and Bard BD; and is a Scientific Advisory Board Member for Medtronic and Xeltis. Dr St. Hilaire has received support from National Institutes of Health grants HL142932 and HL117917. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures







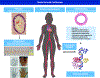
References
-
- Reesink KD, Spronck B. Constitutive interpretation of arterial stiffness in clinical studies: A methodological review. Am J Physiol Heart Circ Physiol 2019;316:H693–H709. - PubMed
-
- Mowafy KA, Soliman M, Hammoda AM, Soliman RM. Bilateral lower limb disabling claudication in a young man: A case of Mönckeberg’s arteriosclerosis. Vasc Endovasc Rev https://www.verjournal.com/articles/Disabling-Claudication-Monckebergs-A... (June 6, 2020).
-
- Pisani I, De Troia A, Allegri L, Corradi D, Vaglio A. Malignant Mönckeberg medial calcific sclerosis. Intern Emerg Med 2018;13:615–617. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

