A Longitudinal Analysis of Early Lesion Growth in Presymptomatic Patients with Cerebral Adrenoleukodystrophy
- PMID: 34503945
- PMCID: PMC8562733
- DOI: 10.3174/ajnr.A7250
A Longitudinal Analysis of Early Lesion Growth in Presymptomatic Patients with Cerebral Adrenoleukodystrophy
Abstract
Background and purpose: Cerebral adrenoleukodystrophy is a devastating neurological disorder caused by mutations in the ABCD1 gene. Our aim was to model and compare the growth of early cerebral lesions from longitudinal MRIs obtained in presymptomatic patients with progressive and arrested cerebral adrenoleukodystrophy using quantitative MR imaging-based lesion volumetry.
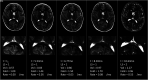
Materials and methods: We retrospectively quantified and modeled the longitudinal growth of early cerebral lesions from 174 MRIs obtained from 36 presymptomatic male patients with cerebral adrenoleukodystrophy. Lesions were manually segmented using subject-specific lesion-intensity thresholding. Volumes were calculated and plotted across time. Lesion velocity and acceleration were calculated between sequentially paired and triplet MRIs, respectively. Linear mixed-effects models were used to assess differences in growth parameters between progressive and arrested phenotypes.
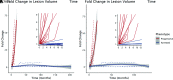
Results: The median patient age was 7.4 years (range, 3.9-37.0 years). Early-stage cerebral disease progression was inversely correlated with age (ρ = -0.6631, P < .001), early lesions can grow while appearing radiographically stable, lesions undergo sustained acceleration in progressive cerebral adrenoleukodystrophy (β = 0.10 mL/month2 [95% CI, 0.05-0.14 mL/month2], P < .001), and growth trajectories diverge between phenotypes in the presymptomatic time period.
Conclusions: Measuring the volumetric changes in newly developing cerebral lesions across time can distinguish cerebral adrenoleukodystrophy phenotypes before symptom onset. When factored into the overall clinical presentation of a patient with a new brain lesion, quantitative MR imaging-based lesion volumetry may aid in the accurate prediction of patients eligible for therapy.
© 2021 by American Journal of Neuroradiology.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
