Cytokine receptor cluster size impacts its endocytosis and signaling
- PMID: 34504012
- PMCID: PMC8449393
- DOI: 10.1073/pnas.2024893118
Cytokine receptor cluster size impacts its endocytosis and signaling
Abstract
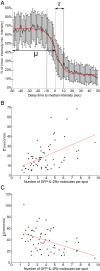
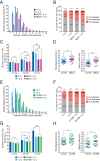
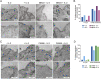
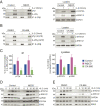
The interleukin-2 receptor (IL-2R) is a cytokine receptor essential for immunity that transduces proliferative signals regulated by its uptake and degradation. IL-2R is a well-known marker of clathrin-independent endocytosis (CIE), a process devoid of any coat protein, raising the question of how the CIE vesicle is generated. Here, we investigated the impact of IL-2Rγ clustering in its endocytosis. Combining total internal reflection fluorescence (TIRF) live imaging of a CRISPR-edited T cell line endogenously expressing IL-2Rγ tagged with green fluorescent protein (GFP), with multichannel imaging, single-molecule tracking, and quantitative analysis, we were able to decipher IL-2Rγ stoichiometry at the plasma membrane in real time. We identified three distinct IL-2Rγ cluster populations. IL-2Rγ is secreted to the cell surface as a preassembled small cluster of three molecules maximum, rapidly diffusing at the plasma membrane. A medium-sized cluster composed of four to six molecules is key for IL-2R internalization and is promoted by interleukin 2 (IL-2) binding, while larger clusters (more than six molecules) are static and inefficiently internalized. Moreover, we identified membrane cholesterol and the branched actin cytoskeleton as key regulators of IL-2Rγ clustering and IL-2-induced signaling. Both cholesterol depletion and Arp2/3 inhibition lead to the assembly of large IL-2Rγ clusters, arising from the stochastic interaction of receptor molecules in close correlation with their enhanced lateral diffusion at the membrane, thus resulting in a default in IL-2R endocytosis. Despite similar clustering outcomes, while cholesterol depletion leads to a sustained IL-2-dependent signaling, Arp2/3 inhibition prevents signal initiation. Taken together, our results reveal the importance of cytokine receptor clustering for CIE initiation and signal transduction.
Keywords: clathrin-independent endocytosis; clustering; signaling; single molecule; stoichiometry.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

