Predicting Symptom Onset in Sporadic Alzheimer Disease With Amyloid PET
- PMID: 34504028
- PMCID: PMC8610624
- DOI: 10.1212/WNL.0000000000012775
Predicting Symptom Onset in Sporadic Alzheimer Disease With Amyloid PET
Abstract
Background and objectives: To predict when cognitively normal individuals with brain amyloidosis will develop symptoms of Alzheimer disease (AD).
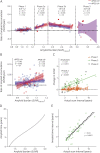
Methods: Brain amyloid burden was measured by amyloid PET with Pittsburgh compound B. The mean cortical standardized uptake value ratio (SUVR) was transformed into a timescale with the use of longitudinal data.
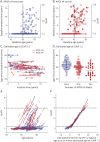
Results: Amyloid accumulation was evaluated in 236 individuals who underwent >1 amyloid PET scan. The average age was 66.5 ± 9.2 years, and 12 individuals (5%) had cognitive impairment at their baseline amyloid PET scan. A tipping point in amyloid accumulation was identified at a low level of amyloid burden (SUVR 1.2), after which nearly all individuals accumulated amyloid at a relatively consistent rate until reaching a high level of amyloid burden (SUVR 3.0). The average time between levels of amyloid burden was used to estimate the age at which an individual reached SUVR 1.2. Longitudinal clinical diagnoses for 180 individuals were aligned by the estimated age at SUVR 1.2. In the 22 individuals who progressed from cognitively normal to a typical AD dementia syndrome, the estimated age at which an individual reached SUVR 1.2 predicted the age at symptom onset (R 2 = 0.54, p < 0.0001, root mean square error [RMSE] 4.5 years); the model was more accurate after exclusion of 3 likely misdiagnoses (R 2 = 0.84, p < 0.0001, RMSE 2.8 years).
Conclusion: The age at symptom onset in sporadic AD is strongly correlated with the age at which an individual reaches a tipping point in amyloid accumulation.
© 2021 American Academy of Neurology.
Figures

References
-
- Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357-367. - PubMed
-
- Harper JD, Lansbury PT Jr. Models of amyloid seeding in Alzheimer's disease and scrapie: mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu Rev Biochem. 1997;66:385-407. - PubMed
-
- Morel B, Conejero-Lara F. Early mechanisms of amyloid fibril nucleation in model and disease-related proteins. Biochim Biophys Acta Proteins Proteom. 2019;1867(11):140264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
