Chemogenetic modulation of histaminergic neurons in the tuberomamillary nucleus alters territorial aggression and wakefulness
- PMID: 34504120
- PMCID: PMC8429727
- DOI: 10.1038/s41598-021-95497-3
Chemogenetic modulation of histaminergic neurons in the tuberomamillary nucleus alters territorial aggression and wakefulness
Abstract
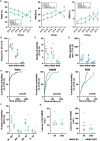
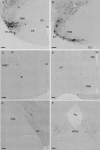
Designer receptor activated by designer drugs (DREADDs) techniques are widely used to modulate the activities of specific neuronal populations during behavioural tasks. However, DREADDs-induced modulation of histaminergic neurons in the tuberomamillary nucleus (HATMN neurons) has produced inconsistent effects on the sleep-wake cycle, possibly due to the use of Hdc-Cre mice driving Cre recombinase and DREADDs activity outside the targeted region. Moreover, previous DREADDs studies have not examined locomotor activity and aggressive behaviours, which are also regulated by brain histamine levels. In the present study, we investigated the effects of HATMN activation and inhibition on the locomotor activity, aggressive behaviours and sleep-wake cycle of Hdc-Cre mice with minimal non-target expression of Cre-recombinase. Chemoactivation of HATMN moderately enhanced locomotor activity in a novel open field. Activation of HATMN neurons significantly enhanced aggressive behaviour in the resident-intruder test. Wakefulness was increased and non-rapid eye movement (NREM) sleep decreased for an hour by HATMN chemoactivation. Conversely HATMN chemoinhibition decreased wakefulness and increased NREM sleep for 6 h. These changes in wakefulness induced by HATMN modulation were related to the maintenance of vigilance state. These results indicate the influences of HATMN neurons on exploratory activity, territorial aggression, and wake maintenance.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

