Characterisation of lamina I anterolateral system neurons that express Cre in a Phox2a-Cre mouse line
- PMID: 34504158
- PMCID: PMC8429737
- DOI: 10.1038/s41598-021-97105-w
Characterisation of lamina I anterolateral system neurons that express Cre in a Phox2a-Cre mouse line
Abstract
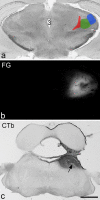
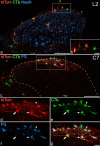
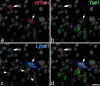
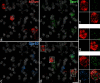
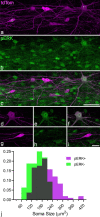
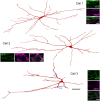
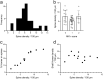
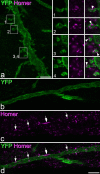
A recently developed Phox2a::Cre mouse line has been shown to capture anterolateral system (ALS) projection neurons. Here, we used this line to test whether Phox2a-positive cells represent a distinct subpopulation among lamina I ALS neurons. We show that virtually all lamina I Phox2a cells can be retrogradely labelled from injections targeted on the lateral parabrachial area (LPb), and that most of those in the cervical cord also belong to the spinothalamic tract. Phox2a cells accounted for ~ 50-60% of the lamina I cells retrogradely labelled from LPb or thalamus. Phox2a was preferentially associated with smaller ALS neurons, and with those showing relatively weak neurokinin 1 receptor expression. The Phox2a cells were also less likely to project to the ipsilateral LPb. Although most Phox2a cells phosphorylated extracellular signal-regulated kinases following noxious heat stimulation, ~ 20% did not, and these were significantly smaller than the activated cells. This suggests that those ALS neurons that respond selectively to skin cooling, which have small cell bodies, may be included among the Phox2a population. Previous studies have defined neurochemical populations among the ALS cells, based on expression of Tac1 or Gpr83. However, we found that the proportions of Phox2a cells that expressed these genes were similar to the proportions reported for all lamina I ALS neurons, suggesting that Phox2a is not differentially expressed among cells belonging to these populations. Finally, we used a mouse line that resulted in membrane labelling of the Phox2a cells and showed that they all possess dendritic spines, although at a relatively low density. However, the distribution of the postsynaptic protein Homer revealed that dendritic spines accounted for a minority of the excitatory synapses on these cells. Our results confirm that Phox2a-positive cells in lamina I are ALS neurons, but show that the Phox2a::Cre line preferentially captures specific types of ALS cells.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

