Genomic landscape of gliosarcoma: distinguishing features and targetable alterations
- PMID: 34504233
- PMCID: PMC8429571
- DOI: 10.1038/s41598-021-97454-6
Genomic landscape of gliosarcoma: distinguishing features and targetable alterations
Abstract
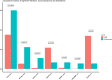
Gliosarcoma is an aggressive brain tumor with histologic features of glioblastoma (GBM) and soft tissue sarcoma. Despite its poor prognosis, its rarity has precluded analysis of its underlying biology. We used a multi-center database to characterize the genomic landscape of gliosarcoma. Sequencing data was obtained from 35 gliosarcoma patients from Genomics Evidence Neoplasia Information Exchange (GENIE) 5.0, a database curated by the American Association of Cancer Research (AACR). We analyzed genomic alterations in gliosarcomas and compared them to GBM (n = 1,449) and soft tissue sarcoma (n = 1,042). 30 samples were included (37% female, median age 59 [IQR: 49-64]). Nineteen common genes were identified in gliosarcoma, defined as those altered in > 5% of samples, including TERT Promoter (92%), PTEN (66%), and TP53 (60%). Of the 19 common genes in gliosarcoma, 6 were also common in both GBM and soft tissue sarcoma, 4 in GBM alone, 0 in soft tissue sarcoma alone, and 9 were more distinct to gliosarcoma. Of these, BRAF harbored an OncoKB level 1 designation, indicating its status as a predictive biomarker of response to an FDA-approved drug in certain cancers. EGFR, CDKN2A, NF1, and PTEN harbored level 4 designations in solid tumors, indicating biological evidence of these biomarkers predicting a drug-response. Gliosarcoma contains molecular features that overlap GBM and soft tissue sarcoma, as well as its own distinct genomic signatures. This may play a role in disease classification and inclusion criteria for clinical trials. Gliosarcoma mutations with potential therapeutic indications include BRAF, EGFR, CDKN2A, NF1, and PTEN.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Blumenthal DT, et al. A phase III study of radiation therapy (RT) and O(6)-benzylguanine + BCNU versus RT and BCNU alone and methylation status in newly diagnosed glioblastoma and gliosarcoma: Southwest Oncology Group (SWOG) study S0001. Int. J. Clin. Oncol. 2015;20:650–658. doi: 10.1007/s10147-014-0769-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

