Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial
- PMID: 34504336
- PMCID: PMC8604729
- DOI: 10.1038/s41591-021-01488-2
Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial
Erratum in
-
Author Correction: Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial.Nat Med. 2022 Jan;28(1):212. doi: 10.1038/s41591-021-01667-1. Nat Med. 2022. PMID: 35022578 Free PMC article. No abstract available.
Abstract
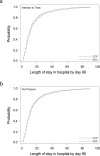
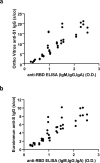
The efficacy of convalescent plasma for coronavirus disease 2019 (COVID-19) is unclear. Although most randomized controlled trials have shown negative results, uncontrolled studies have suggested that the antibody content could influence patient outcomes. We conducted an open-label, randomized controlled trial of convalescent plasma for adults with COVID-19 receiving oxygen within 12 d of respiratory symptom onset ( NCT04348656 ). Patients were allocated 2:1 to 500 ml of convalescent plasma or standard of care. The composite primary outcome was intubation or death by 30 d. Exploratory analyses of the effect of convalescent plasma antibodies on the primary outcome was assessed by logistic regression. The trial was terminated at 78% of planned enrollment after meeting stopping criteria for futility. In total, 940 patients were randomized, and 921 patients were included in the intention-to-treat analysis. Intubation or death occurred in 199/614 (32.4%) patients in the convalescent plasma arm and 86/307 (28.0%) patients in the standard of care arm-relative risk (RR) = 1.16 (95% confidence interval (CI) 0.94-1.43, P = 0.18). Patients in the convalescent plasma arm had more serious adverse events (33.4% versus 26.4%; RR = 1.27, 95% CI 1.02-1.57, P = 0.034). The antibody content significantly modulated the therapeutic effect of convalescent plasma. In multivariate analysis, each standardized log increase in neutralization or antibody-dependent cellular cytotoxicity independently reduced the potential harmful effect of plasma (odds ratio (OR) = 0.74, 95% CI 0.57-0.95 and OR = 0.66, 95% CI 0.50-0.87, respectively), whereas IgG against the full transmembrane spike protein increased it (OR = 1.53, 95% CI 1.14-2.05). Convalescent plasma did not reduce the risk of intubation or death at 30 d in hospitalized patients with COVID-19. Transfusion of convalescent plasma with unfavorable antibody profiles could be associated with worse clinical outcomes compared to standard care.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures










Comment in
-
Reply to: Concerns about estimating relative risk of death associated with convalescent plasma for COVID-19.Nat Med. 2022 Jan;28(1):53-58. doi: 10.1038/s41591-021-01639-5. Epub 2022 Jan 10. Nat Med. 2022. PMID: 35013612 No abstract available.
-
Concerns about estimating relative risk of death associated with convalescent plasma for COVID-19.Nat Med. 2022 Jan;28(1):51-52. doi: 10.1038/s41591-021-01638-6. Epub 2022 Jan 10. Nat Med. 2022. PMID: 35013614 No abstract available.
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

