Tofacitinib protects intestinal epithelial cells against oxygen-glucose deprivation/reoxygenation injury by inhibiting the JAK/STAT3 signaling pathway
- PMID: 34504562
- PMCID: PMC8383770
- DOI: 10.3892/etm.2021.10542
Tofacitinib protects intestinal epithelial cells against oxygen-glucose deprivation/reoxygenation injury by inhibiting the JAK/STAT3 signaling pathway
Abstract
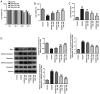
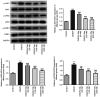
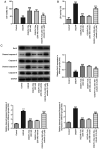
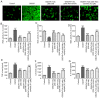
The present study aimed to investigate the role and potential mechanism of action of tofacitinib (Tofa) in intestinal ischemia/reperfusion (I/R) injury. The normal rat small intestine epithelial cell line, IEC-6, was used to establish an I/R injury model by inducing oxygen-glucose deprivation/reoxygenation (OGD/R). Cells were divided into the following five groups: Control, OGD/R, OGD/R with 50, 100 and 200 nM Tofa. Following Tofa administration, cell viability was measured using Cell Counting Kit-8 assay and a lactate dehydrogenase detection kit. The expression levels of cell apoptosis-related proteins, Bcl-2, cleaved-caspase-3 and cleaved-caspase-9 were detected using western blot analysis. Additionally, the levels of oxidative stress-related markers, such as reactive oxygen species (ROS), malondialdehyde (MDA) and superoxide dismutase (SOD), and inflammatory cytokines, TNF-α, IL-6 and IL-1β were assessed using the colorimetric method. Western blot analysis was also used to measure the expression levels of the Janus kinase (JAK)/STAT3 signaling pathway-related proteins, including phosphorylated (p)-JAK1, p-JAK3 and p-STAT3. Subsequently, colivelin, an agonist of the JAK/STAT3 pathway, was used to investigate whether the effects of Tofa on intestinal I/R injury were mediated by this signaling pathway. The results showed that Tofa dose-dependently elevated cell viability compared with that in the OGD/R group. By contrast, Tofa attenuated cell apoptosis, which was coupled with upregulated Bcl-2 expression, downregulated cleaved-caspase-3 and downregulated cleaved-caspase-9 levels, in OGD/R-induced IEC-6 cells. Furthermore, the contents of ROS and MDA were significantly increased following exposure to OGD/R, which were accompanied by the decreased activity of SOD. These effects were reversed following cell treatment with Tofa. Consistently, Tofa intervention reduced the secretion levels of TNF-α, IL-6 and IL-1β in a dose-dependent manner. Additionally, Tofa markedly downregulated the phosphorylation levels of JAK1, JAK3 and STAT3 in OGD/R-induced IEC-6 cells. However, treatment with colivelin markedly reversed the inhibitory effects of Tofa on cell viability, cell apoptosis, oxidative stress and inflammation. Overall, the findings of the present study suggested that Tofa could protect against intestinal I/R injury by inhibiting the JAK/STAT3 signaling pathway, which may hold promise as a therapeutic agent for intestinal I/R injury.
Keywords: Janus kinase; apoptosis; inflammation; intestinal ischemia/reperfusion; oxidative stress.
Copyright: © Yang et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Paeoniflorin protects PC12 cells from oxygen-glucose deprivation/reoxygenation-induced injury via activating JAK2/STAT3 signaling.Exp Ther Med. 2021 Jun;21(6):572. doi: 10.3892/etm.2021.10004. Epub 2021 Mar 29. Exp Ther Med. 2021. PMID: 33850544 Free PMC article.
-
Astragaloside IV Alleviates Cerebral Ischemia-Reperfusion Injury by Activating the Janus Kinase 2 and Signal Transducer and Activator of Transcription 3 Signaling Pathway.Pharmacology. 2020;105(3-4):181-189. doi: 10.1159/000503361. Epub 2019 Dec 11. Pharmacology. 2020. PMID: 31825924
-
Overexpression of MiR-188-5p Downregulates IL6ST/STAT3/ NLRP3 Pathway to Ameliorate Neuron Injury in Oxygen-glucose Deprivation/Reoxygenation.Curr Neurovasc Res. 2024;21(3):263-273. doi: 10.2174/0115672026313555240515103132. Curr Neurovasc Res. 2024. PMID: 38778610
-
Oxidative Stress in Intestinal Ischemia-Reperfusion.Front Med (Lausanne). 2022 Jan 14;8:750731. doi: 10.3389/fmed.2021.750731. eCollection 2021. Front Med (Lausanne). 2022. PMID: 35096858 Free PMC article. Review.
-
The Interaction Between Autophagy and JAK/STAT3 Signaling Pathway in Tumors.Front Genet. 2022 Apr 26;13:880359. doi: 10.3389/fgene.2022.880359. eCollection 2022. Front Genet. 2022. PMID: 35559037 Free PMC article. Review.
Cited by
-
Effect of tofacitinib on the phenotype and activity of Caco‑2 cells in a model of inflammatory bowel disease.Exp Ther Med. 2024 Feb 20;27(4):152. doi: 10.3892/etm.2024.12440. eCollection 2024 Apr. Exp Ther Med. 2024. PMID: 38476894 Free PMC article.
-
Stimulatory Effect of Tofacitinib on Bone Marrow Adipocytes Differentiation.Front Endocrinol (Lausanne). 2022 Jul 6;13:881699. doi: 10.3389/fendo.2022.881699. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35873000 Free PMC article.
References
-
- Leone M, Bechis C, Baumstarck K, Ouattara A, Collange O, Augustin P, Annane D, Arbelot C, Asehnoune K, Baldési O, et al. Outcome of acute mesenteric ischemia in the intensive care unit: A retrospective, multicenter study of 780 cases. Intensive Care Med. 2015;41:667–676. doi: 10.1007/s00134-015-3690-8. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
