Imaging of Cardiac Device-Related Infection
- PMID: 34504881
- PMCID: PMC8421771
- DOI: 10.3389/fcvm.2021.729786
Imaging of Cardiac Device-Related Infection
Abstract
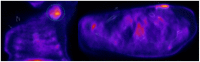
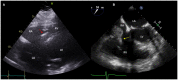
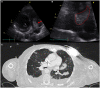
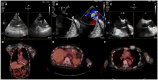
Cardiac devices are frequently used in different cardiovascular conditions for the purpose of morbidity or mortality prevention. These include cardiac implantable electronic devices (CIED) like permanent pacemakers and implantable cardiac defibrillators, ventricular assistance devices (VADs), left atrial appendage occlusion (LAAO) devices like the Watchman™, atrial and ventricular septal occluders like the Amplatzer™, among others. In the past years, there has been an increase in the development of these devices as a result of a rise in the number of indications for implantation, paired with the aging and more medically complex patient population. This has led to an increase in the incidence of cardiac device-related infections, one of the most feared and serious complications which is associated with significant morbidity, mortality and financial burden. Accurate diagnosis of cardiac device-related infections is essential given the management implications which often involve removal of the infected device, removal of other prosthetic material and long-term antimicrobial therapy. Clinical and laboratory data are useful diagnostic tools but multimodality imaging is often necessary. The recently published 2020 European Heart Rhythm Association International Consensus document, which is endorsed by many expert societies, has recommended the use of multimodality imaging for the diagnosis of CIED infections. (1) This allows better disease characterization by identifying abnormal fluid collections and guiding aspiration for both diagnostic and therapeutic purposes (i.e. soft tissue ultrasound and computed tomography), evaluation for local extent of disease (i.e. transesophageal echocardiogram to evaluate for concomitant infective endocarditis), embolic manifestation of disease (i.e. computed tomography and magnetic resonance imaging) and metabolic tissue characterization (positron emission tomography and tagged white blood cell scan). (2) In addition, computed tomography (CT) allows for pre-procedural planning which has shown to be associated with better procedural outcomes.
Keywords: cardiac device; cardiac implantable electronic devices; computed tomography; device infection; endocarditis; left ventricular assist devices; positron emission tomography; transesophageal echocardiogram.
Copyright © 2021 Aguilera, Hutt and Jaber.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
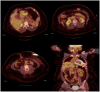
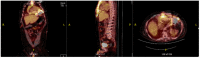
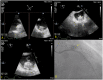
Similar articles
-
European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS).Eur J Cardiothorac Surg. 2020 Jan 1;57(1):e1-e31. doi: 10.1093/ejcts/ezz296. Eur J Cardiothorac Surg. 2020. PMID: 31724720
-
European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS).Europace. 2020 Apr 1;22(4):515-549. doi: 10.1093/europace/euz246. Europace. 2020. PMID: 31702000 Free PMC article.
-
New diagnostic pathways urgently needed. Protocol of PET Guidance I pilot study: positron emission tomography in suspected cardiac implantable electronic device-related infection.Kardiol Pol. 2016;74(1):47-52. doi: 10.5603/KP.a2015.0113. Epub 2015 Jun 23. Kardiol Pol. 2016. PMID: 26101020
-
Cardiovascular Imaging in Infective Endocarditis: A Multimodality Approach.Circ Cardiovasc Imaging. 2020 Jul;13(7):e008956. doi: 10.1161/CIRCIMAGING.120.008956. Epub 2020 Jul 20. Circ Cardiovasc Imaging. 2020. PMID: 32683888 Review.
-
Single Photon Emission Tomography in the Diagnostic Assessment of Cardiac and Vascular Infectious Diseases.Curr Radiopharm. 2021;14(3):242-258. doi: 10.2174/1874471013666200621190001. Curr Radiopharm. 2021. PMID: 32564768 Review.
Cited by
-
Native valve, prosthetic valve, and cardiac device-related infective endocarditis: A review and update on current innovative diagnostic and therapeutic strategies.Front Cell Dev Biol. 2022 Oct 3;10:995508. doi: 10.3389/fcell.2022.995508. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36263017 Free PMC article. Review.
-
New Diseases Related to Cardiac Implantable Electronic Devices (CIEDs): An Overview.J Clin Med. 2025 Feb 17;14(4):1322. doi: 10.3390/jcm14041322. J Clin Med. 2025. PMID: 40004852 Free PMC article. Review.
-
Intractable late onset pacemaker endocarditis and complications case report.Heliyon. 2024 Nov 1;10(21):e40073. doi: 10.1016/j.heliyon.2024.e40073. eCollection 2024 Nov 15. Heliyon. 2024. PMID: 39553610 Free PMC article.
-
Relevance of cardiac imaging in the evolving landscape of infective endocarditis management.Ther Adv Cardiovasc Dis. 2024 Jan-Dec;18:17539447241305587. doi: 10.1177/17539447241305587. Ther Adv Cardiovasc Dis. 2024. PMID: 39655905 Free PMC article. Review.
-
Modern tools in cardiac imaging to assess myocardial inflammation and infection.Eur Heart J Open. 2023 Mar 3;3(2):oead019. doi: 10.1093/ehjopen/oead019. eCollection 2023 Mar. Eur Heart J Open. 2023. PMID: 37006410 Free PMC article. Review.
References
-
- Blomström-Lundqvist C, Traykov V, Erba PA, Burri H, Nielsen JC, Bongiorni MG, et al. . European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID), and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2020) 41:2012–32. 10.1093/eurheartj/ehaa010 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

