In-vivo sub-diffraction adaptive optics imaging of photoreceptors in the human eye with annular pupil illumination and sub-Airy detection
- PMID: 34504903
- PMCID: PMC8425240
- DOI: 10.1364/optica.414206
In-vivo sub-diffraction adaptive optics imaging of photoreceptors in the human eye with annular pupil illumination and sub-Airy detection
Abstract
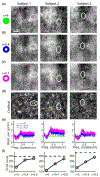
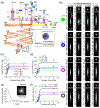
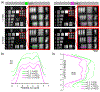
Adaptive optics scanning light ophthalmoscopy (AOSLO) allows non-invasive visualization of the living human eye at the microscopic scale; but even with correction of the ocular wavefront aberrations over a large pupil, the smallest cells in the photoreceptor mosaic cannot always be resolved. Here, we synergistically combine annular pupil illumination with sub-Airy disk confocal detection to demonstrate a 33% improvement in transverse resolution (from 2.36 to 1.58 μm) and a 13% axial resolution enhancement (from 37 to 32 μm), an important step towards the study of the complete photoreceptor mosaic in heath and disease. Interestingly, annular pupil illumination also enhanced the visualization of the photoreceptor mosaic in non-confocal detection schemes such as split detection AOSLO, providing a strategy for enhanced multimodal imaging of the cone and rod photoreceptor mosaic.
Conflict of interest statement
Disclosures. The authors declare no conflict of interests.
Figures



References
-
- Curcio CA, Sloan KR, Kalina RE, and Hendrickson AE, “Human photoreceptor topography,” J. Comparative Neurol 292, 497–523 (1990). - PubMed
-
- Webb RH, Hughes GW, and Delori FC, “Confocal scanning laser ophthalmoscope,” Appl. Opt 26,1492–1499 (1987). - PubMed
-
- Elsner AE, Burns SA, Hughes GW, and Webb RH, “Reflectometry with a scanning laser ophthalmoscope,” Appl. Opt 31, 3697–3710 (1992). - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
