A prospective observational safety study on ChAdOx1 nCoV-19 corona virus vaccine (recombinant) use in healthcare workers- first results from India
- PMID: 34505032
- PMCID: PMC8413251
- DOI: 10.1016/j.eclinm.2021.101038
A prospective observational safety study on ChAdOx1 nCoV-19 corona virus vaccine (recombinant) use in healthcare workers- first results from India
Abstract
Background: We provide the first post-approval safety analysis of COVISHIELD in health care workers (HCWs) in northern India.
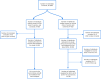
Methods: This continuing prospective observational study (February 2021 to May 2022) enrolled participants ≥18 years receiving COVISHIELD vaccination. Primary outcome was safety and reactogenicity. Categories (FDA toxicity grading) and outcomes of adverse events following immunization (AEFIs) were recorded, causality assessment performed, and risk factors analysed.
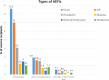
Findings: We present the results of an interim analysis of 804 participants. AEFIs following first dose were reported in 321 (40%; systemic involvement in 248). Among 730 participants who completed a 7-day follow-up post second dose, AEFIs occurred in 115 (15.7%; systemic in 99). Majority of AEFIs were mild-moderate and resolved spontaneously. Serious AEFIs, leading to hospitalization was noticed in 1 (0.1%) participant with suspicion of immunization stress related response (ISRR). AEFIs of grade 3 severity (FDA) were recorded in 4 participants (0.5%). No deaths were recorded. Regression analysis showed increased risk of AEFIs in younger individuals, a two times higher odds in females, those with hypertension or with history of allergy; and three times higher odds in individuals with hypothyroidism.
Interpretation: COVISHIELD carries an overall favourable safety profile with AEFI rates much less than reported for other adenoviral vaccines. Females, those with hypertension, individuals with history of allergy and hypothyroidism may need watchful vaccine administration. This being an interim analysis and based on healthcare workers who may not reflect the general population demographics, larger inclusive studies are warranted for confirming the findings.
Funding: No funding support.
Keywords: AEFI; COVID-19; India; Post-approval; Risk; Thyroid.
© 2021 The Author(s).
Conflict of interest statement
The authors have nothing to declare.
Figures

References
-
- U.S. Food and Drug Administration. COVID-19 vaccines. 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise... (accessed July 5, 2021).
LinkOut - more resources
Full Text Sources

