ALBI grade: Evidence for an improved model for liver functional estimation in patients with hepatocellular carcinoma
- PMID: 34505035
- PMCID: PMC8411239
- DOI: 10.1016/j.jhepr.2021.100347
ALBI grade: Evidence for an improved model for liver functional estimation in patients with hepatocellular carcinoma
Abstract
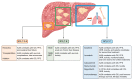
Hepatocellular carcinoma (HCC) usually arises in the context of a chronically damaged liver. Liver functional estimation is of paramount importance in clinical decision making. The Child-Pugh score (CPS) can be used to categorise patients into 3 classes (A to C) based on the severity of liver functional impairment according to 5 parameters (albumin, bilirubin, prothrombin time, presence of ascites and hepatic encephalopathy). The albumin-bilirubin (ALBI) grade has emerged as an alternative, reproducible and objective measure of liver functional reserve in patients with HCC, defining worsening liver impairment across 3 grades (I to III). The ALBI score can identify different subgroups of patients with different prognoses across the diverse Barcelona Clinic Liver Cancer stages and CP classes, making it an appealing clinical predictor. In patients treated with potentially curative approaches (resection, transplantation, radiofrequency ablation, microwave ablation), ALBI grade has been shown to correlate with survival, tumour relapse, and post-hepatectomy liver failure. ALBI grade also predicts survival, toxicity and post-procedural liver failure in patients treated with transarterial chemoembolisation, radioembolisation, external beam radiotherapy as well as multi-kinase inhibitors (sorafenib, lenvatinib, cabozantinib, regorafenib) and immune checkpoint inhibitor therapy. In this review, we summarise the body of evidence surrounding the role of ALBI grade as a biomarker capable of optimising patient selection and therapeutic sequencing in HCC.
Keywords: ALBI, albumin-bilirubin; APRI, aspartate aminotransferase to platelet count index; BCLC, Barcelona Clinic Liver Cancer; CLD, chronic liver disease; CPS, Child-Pugh score; Child-Pugh; HCC; HCC, hepatocellular carcinoma; ICIs, immune checkpoint inhibitors; LT, liver transplantation; MELD, model for end-stage liver disease; ORR, objective response rate; OS, overall survival; PHLF, post-hepatectomy liver failure; RFS, recurrence-free survival; TACE, transarterial chemoembolisation; TARE, transarterial radioembolisation; cirrhosis; liver function; mAb, monoclonal antibody; prognosis.
© 2021 The Authors.
Conflict of interest statement
DJP received lecture fees from ViiV Healthcare and Bayer Healthcare and travel expenses from BMS and Bayer Healthcare; consulting fees for Mina Therapeutics, EISAI, Roche, and Astra Zeneca; received research funding (to institution) from MSD and BMS. RS received consulting fees for EISAI, Roche, Bayer, SIRTEX, Novartis; research funding (to institution) from Incyte, Novartis, Astex Pharmaceuticals, Bayer and Boston Scientific. LR received consulting fees from Amgen, ArQule, AstraZeneca, Basilea, Bayer, BMS, Celgene, Eisai, Exelixis, Genenta, Hengrui, Incyte, Ipsen, IQVIA, Lilly, MSD, Nerviano Medical Sciences, Roche, Sanofi, Zymeworks; lecture fees from AbbVie, Amgen, Bayer, Eisai, Gilead, Incyte, Ipsen, Lilly, Merck Serono, Roche, Sanofi; travel expenses from Ipsen; and institutional research funding from Agios, ARMO BioSciences, AstraZeneca, BeiGene, Eisai, Exelixis, Fibrogen, Incyte, Ipsen, Lilly, MSD, Nerviano Medical Sciences, Roche, Zymeworks. All remaining authors have declared no conflicts of interest. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilised in the production of this manuscript. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures
References
-
- Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D.M., Pineros M. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Canc. 2019;144(8):1941–1953. - PubMed
-
- Morgan E.T., Goralski K.B., Piquette-Miller M., Renton K.W., Robertson G.R., Chaluvadi M.R. Regulation of drug-metabolizing enzymes and transporters in infection, inflammation, and cancer. Drug Metab Dispos. 2008;36(2):205–216. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

