Tie2 activation protects against prothrombotic endothelial dysfunction in COVID-19
- PMID: 34506304
- PMCID: PMC8564889
- DOI: 10.1172/jci.insight.151527
Tie2 activation protects against prothrombotic endothelial dysfunction in COVID-19
Abstract
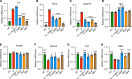
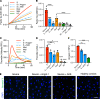
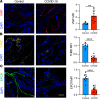
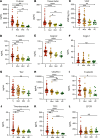
Endothelial dysfunction accompanies the microvascular thrombosis commonly observed in severe COVID-19. Constitutively, the endothelial surface is anticoagulant, a property maintained at least in part via signaling through the Tie2 receptor. During inflammation, the Tie2 antagonist angiopoietin-2 (Angpt-2) is released from endothelial cells and inhibits Tie2, promoting a prothrombotic phenotypic shift. We sought to assess whether severe COVID-19 is associated with procoagulant endothelial dysfunction and alterations in the Tie2/angiopoietin axis. Primary HUVECs treated with plasma from patients with severe COVID-19 upregulated the expression of thromboinflammatory genes, inhibited the expression of antithrombotic genes, and promoted coagulation on the endothelial surface. Pharmacologic activation of Tie2 with the small molecule AKB-9778 reversed the prothrombotic state induced by COVID-19 plasma in primary endothelial cells. Lung autopsies from patients with COVID-19 demonstrated a prothrombotic endothelial signature. Assessment of circulating endothelial markers in a cohort of 98 patients with mild, moderate, or severe COVID-19 revealed endothelial dysfunction indicative of a prothrombotic state. Angpt-2 concentrations rose with increasing disease severity, and the highest levels were associated with worse survival. These data highlight the disruption of Tie2/angiopoietin signaling and procoagulant changes in endothelial cells in severe COVID-19. Our findings provide rationale for current trials of Tie2-activating therapy with AKB-9778 in COVID-19.
Keywords: COVID-19; Coagulation; Vascular Biology.
Conflict of interest statement
Figures
Update of
-
Tie2 activation protects against prothrombotic endothelial dysfunction in COVID-19.medRxiv [Preprint]. 2021 May 17:2021.05.13.21257070. doi: 10.1101/2021.05.13.21257070. medRxiv. 2021. Update in: JCI Insight. 2021 Oct 22;6(20):e151527. doi: 10.1172/jci.insight.151527. PMID: 34031665 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

