Optimal Timing of Administration of Direct-acting Antivirals for Patients With Hepatitis C-associated Hepatocellular Carcinoma Undergoing Liver Transplantation
- PMID: 34506316
- PMCID: PMC8559662
- DOI: 10.1097/SLA.0000000000005070
Optimal Timing of Administration of Direct-acting Antivirals for Patients With Hepatitis C-associated Hepatocellular Carcinoma Undergoing Liver Transplantation
Abstract
Objective: To investigate the optimal timing of direct acting antiviral (DAA) administration in patients with hepatitis C-associated hepatocellular carcinoma (HCC) undergoing liver transplantation (LT).
Summary of background data: In patients with hepatitis C (HCV) associated HCC undergoing LT, the optimal timing of direct-acting antivirals (DAA) administration to achieve sustained virologic response (SVR) and improved oncologic outcomes remains a topic of much debate.
Methods: The United States HCC LT Consortium (2015-2019) was reviewed for patients with primary HCV-associated HCC who underwent LT and received DAA therapy at 20 institutions. Primary outcomes were SVR and HCC recurrence-free survival (RFS).
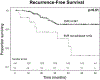
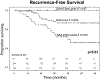
Results: Of 857 patients, 725 were within Milan criteria. SVR was associated with improved 5-year RFS (92% vs 77%, P < 0.01). Patients who received DAAs pre-LT, 0-3 months post-LT, and ≥3 months post-LT had SVR rates of 91%, 92%, and 82%, and 5-year RFS of 93%, 94%, and 87%, respectively. Among 427 HCV treatment-naïve patients (no previous interferon therapy), patients who achieved SVR with DAAs had improved 5-year RFS (93% vs 76%, P < 0.01). Patients who received DAAs pre-LT, 0-3 months post-LT, and ≥3 months post-LT had SVR rates of 91%, 93%, and 78% (P < 0.01) and 5-year RFS of 93%, 100%, and 83% (P = 0.01).
Conclusions: The optimal timing of DAA therapy appears to be 0 to 3 months after LT for HCV-associated HCC, given increased rates of SVR and improved RFS. Delayed administration after transplant should be avoided. A prospective randomized controlled trial is warranted to validate these results.
Copyright © 2021 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
S. A. Shah, Veloxis Pharmaceuticals Role(s): Membership on advisory committee or review panels. Organ Recovery Systems Role(s): Other activities (please specify), Research funding. J. Emamaullee, iSPY COVID trial Role(s): Other activities (please specify), Data Monitoring Committee. Gilead Role(s): Other activities (please specify), Research funding. American Society of Transplant Surgeons Role(s): Other activities (please specify), Research funding. American Association for the Study of Liver Diseases Role(s): Other activities (please specify), Research Funding. National Cancer Institute Role(s): Other activities (please specify), Research funding. M. H. Nguyen, Gilead Role(s): Other activities (please specify), Research support. Glycotest Role(s): Other activities (please specify), Research support. National Cancer Institute Role(s): Other activities (please specify), Research support. Bayer Role(s): Consulting. Esai Role(s): Consulting. Novartis Role(s): Consulting. Gilead Role(s): Consulting. Exact Sciences Role(s): Consulting. Laboratory of Advanced Medicine Role(s): Consulting. W. C. Chapman, Novartis Role(s): Membership on advisory committee or review panels. Pathfinder Role(s): Other activities (please specify), IP royalties.; S. K. Maithel, Celgene (Bristol Meyers Squibbs). All the other authors report no conflicts of interest.
Figures
References
-
- Kim HS, El-Serag HB. The epidemiology of hepatocellular carcinoma in the USA. Curr Gastroenterol Rep. 2019;21:17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

