Adherence to community versus facility-based delivery of monthly malaria chemoprevention with dihydroartemisinin-piperaquine for the post-discharge management of severe anemia in Malawian children: A cluster randomized trial
- PMID: 34506503
- PMCID: PMC8432777
- DOI: 10.1371/journal.pone.0255769
Adherence to community versus facility-based delivery of monthly malaria chemoprevention with dihydroartemisinin-piperaquine for the post-discharge management of severe anemia in Malawian children: A cluster randomized trial
Abstract
Background: The provision of post-discharge malaria chemoprevention (PMC) in children recently admitted with severe anemia reduces the risk of death and re-admissions in malaria endemic countries. The main objective of this trial was to identify the most effective method of delivering dihydroartemesinin-piperaquine to children recovering from severe anemia.
Methods: This was a 5-arm, cluster-randomized trial among under-5 children hospitalized with severe anemia at Zomba Central Hospital in Southern Malawi. Children were randomized to receive three day treatment doses of dihydroartemesinin-piperaquine monthly either; 1) in the community without a short text reminder; 2) in the community with a short message reminder; 3) in the community with a community health worker reminder; 4) at the facility without a short text reminder; or 5) at the facility with a short message reminder. The primary outcome measure was adherence to all treatment doses of dihydroartemesinin-piperaquine and this was assessed by pill-counts done by field workers during home visits. Poisson regression was utilized for analysis.
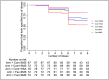
Results: Between March 2016 and October 2018, 1460 clusters were randomized. A total of 667 children were screened and 375 from 329 clusters were eligible and enrolled from the hospital. Adherence was higher in all three community-based compared to the two facility-based delivery (156/221 [70·6%] vs. 78/150 [52·0%], IRR = 1·24,95%CI 1·06-1·44, p = 0·006). This was observed in both the SMS group (IRR = 1·41,1·21-1·64, p<0·001) and in the non-SMS group (IRR = 1·37,1·18-1·61, p<0·001). Although adherence was higher among SMS recipients (98/148 66·2%] vs. non-SMS 82/144 (56·9%), there was no statistical evidence that SMS reminders resulted in greater adherence ([IRR = 1·03,0·88-1·21, p = 0·68). When compared to the facility-based non-SMS arm (control arm), community-based delivery utilizing CHWs resulted in higher adherence [39/76 (51·3%) vs. 54/79 (68·4%), IRR = 1·32, 1·14-1·54, p<0·001].
Interpretation: Community-based delivery of dihydroartemesinin-piperaquine for post-discharge malaria chemoprevention in children recovering from severe anemia resulted in higher adherence compared to facility-based methods.
Trial registration: NCT02721420; ClinicalTrials.gov.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Delivery strategies for malaria chemoprevention with monthly dihydroartemisinin-piperaquine for the post-discharge management of severe anaemia in children aged less than 5 years old in Malawi: a protocol for a cluster randomized trial.BMC Pediatr. 2018 Jul 20;18(1):238. doi: 10.1186/s12887-018-1199-3. BMC Pediatr. 2018. PMID: 30029620 Free PMC article.
-
Introducing post-discharge malaria chemoprevention (PMC) for management of severe anemia in Malawian children: a qualitative study of community health workers' perceptions and motivation.BMC Health Serv Res. 2018 Dec 19;18(1):984. doi: 10.1186/s12913-018-3791-5. BMC Health Serv Res. 2018. PMID: 30567567 Free PMC article. Clinical Trial.
-
Post-discharge malaria chemoprevention (PMC) in Malawi: caregivers` acceptance and preferences with regard to delivery methods.BMC Health Serv Res. 2018 Jul 11;18(1):544. doi: 10.1186/s12913-018-3327-z. BMC Health Serv Res. 2018. PMID: 29996833 Free PMC article. Clinical Trial.
-
Efficacy of dihydroartemisinin-piperaquine versus artemether-lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria among children in Africa: a systematic review and meta-analysis of randomized control trials.Malar J. 2021 Aug 12;20(1):340. doi: 10.1186/s12936-021-03873-1. Malar J. 2021. PMID: 34384431 Free PMC article.
-
Dihydroartemisinin/Piperaquine: a review of its use in the treatment of uncomplicated Plasmodium falciparum malaria.Drugs. 2012 May 7;72(7):937-61. doi: 10.2165/11203910-000000000-00000. Drugs. 2012. PMID: 22515619 Review.
Cited by
-
Paediatric anaemia in rural Kenya and the role of travel time to emergency care services.Front Epidemiol. 2025 May 15;5:1578522. doi: 10.3389/fepid.2025.1578522. eCollection 2025. Front Epidemiol. 2025. PMID: 40444254 Free PMC article.
-
Perceived barriers and opportunities for the introduction of post-discharge malaria chemoprevention (PDMC) in five sub-Saharan countries: a qualitative survey amongst malaria key stakeholders.Malar J. 2024 Sep 6;23(1):270. doi: 10.1186/s12936-024-05100-z. Malar J. 2024. PMID: 39243086 Free PMC article.
-
Economic evaluation of postdischarge malaria chemoprevention in preschool children treated for severe anaemia in Malawi, Kenya, and Uganda: A cost-effectiveness analysis.EClinicalMedicine. 2022 Oct 1;52:101669. doi: 10.1016/j.eclinm.2022.101669. eCollection 2022 Oct. EClinicalMedicine. 2022. PMID: 36313146 Free PMC article.
-
Pediatric post-discharge mortality in resource-poor countries: a systematic review and meta-analysis.EClinicalMedicine. 2023 Dec 21;67:102380. doi: 10.1016/j.eclinm.2023.102380. eCollection 2024 Jan. EClinicalMedicine. 2023. PMID: 38204490 Free PMC article.
-
Implementation of post-discharge malaria chemoprevention (PDMC) in Benin, Kenya, Malawi, and Uganda: stakeholder engagement meeting report.Malar J. 2024 Mar 27;23(1):89. doi: 10.1186/s12936-023-04810-0. Malar J. 2024. PMID: 38539181 Free PMC article.
References
-
- World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System (WHO/NMH/NHD/MNM/11.1). Geneva: World Health Organization; 2011.
-
- World Health Organization. The global prevalence of anaemia in 2011. Geneva: World Health Organization; 2015.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

