Medial prefrontal area reductions, altered expressions of cholecystokinin, parvalbumin, and activating transcription factor 4 in the corticolimbic system, and altered emotional behavior in a progressive rat model of type 2 diabetes
- PMID: 34506507
- PMCID: PMC8432800
- DOI: 10.1371/journal.pone.0256655
Medial prefrontal area reductions, altered expressions of cholecystokinin, parvalbumin, and activating transcription factor 4 in the corticolimbic system, and altered emotional behavior in a progressive rat model of type 2 diabetes
Abstract
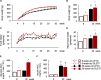
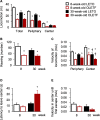
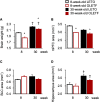
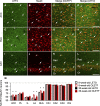
Metabolic disorders are associated with a higher risk of psychiatric disorders. We previously reported that 20-week-old Otsuka Long-Evans Tokushima fatty (OLETF) rats, a model of progressive type 2 diabetes, showed increased anxiety-like behavior and regional area reductions and increased cholecystokinin-positive neurons in the corticolimbic system. However, in which stages of diabetes these alterations in OLETF rats occur remains unclear. We aimed to investigate anxiety-like behavior and its possible mechanisms at different stages of type 2 diabetes in OLETF rats. Eight- and 30-week-old OLETF rats were used as diabetic animal models at the prediabetic and progressive stages of type 2 diabetes respectively, and age-matched Long-Evans Tokushima Otsuka rats served as non-diabetic controls. In the open-field test, OLETF rats showed less locomotion in the center zone and longer latency to leave the center zone at 8 and 30 weeks old, respectively. The areas of the medial prefrontal cortex were smaller in the OLETF rats, regardless of age. The densities of cholecystokinin-positive neurons in OLETF rats were higher in the lateral and basolateral amygdala only at 8 weeks old and in the anterior cingulate and infralimbic cortices and hippocampal cornu ammonis area 3 at both ages. The densities of parvalbumin-positive neurons of OLETF rats were lower in the cornu ammonis area 2 at 8 weeks old and in the prelimbic and infralimbic cortices at both ages. No apoptotic cell death was detected in OLETF rats, but the percentage of neurons co-expressing activating transcription factor 4 and cholecystokinin and parvalbumin was higher in OLETF rats at both ages in the anterior cingulate cortex and basolateral amygdala, respectively. These results suggest that altered emotional behavior and related neurological changes in the corticolimbic system are already present in the prediabetic stage of OLETF rats.
Conflict of interest statement
No authors have competing interests.
Figures



Similar articles
-
Region-specific brain area reductions and increased cholecystokinin positive neurons in diabetic OLETF rats: implication for anxiety-like behavior.J Physiol Sci. 2020 Sep 16;70(1):42. doi: 10.1186/s12576-020-00771-0. J Physiol Sci. 2020. PMID: 32938363 Free PMC article.
-
Voluntary exercise reverses social behavior deficits and the increases in the densities of cholecystokinin-positive neurons in specific corticolimbic regions of diabetic OLETF rats.Behav Brain Res. 2022 Jun 25;428:113886. doi: 10.1016/j.bbr.2022.113886. Epub 2022 Apr 7. Behav Brain Res. 2022. PMID: 35398486
-
Increased anxiety behavior in OLETF rats without cholecystokinin-A receptor.Brain Res Bull. 2000 Dec;53(6):789-92. doi: 10.1016/s0361-9230(00)00407-x. Brain Res Bull. 2000. PMID: 11179844
-
Changes in interleukin 18 in the retinas of Otsuka long-evans Tokushima fatty rats, a model of human type 2 diabetes.J Oleo Sci. 2013;62(7):513-23. doi: 10.5650/jos.62.513. J Oleo Sci. 2013. PMID: 23823918
-
[The biological clock mechanism in non-insulin-dependent and insulin-dependent diabetic rats].Nihon Yakurigaku Zasshi. 2000 Aug;116(2):71-8. doi: 10.1254/fpj.116.71. Nihon Yakurigaku Zasshi. 2000. PMID: 10976458 Review. Japanese.
Cited by
-
Affective and Cognitive Impairments in Rodent Models of Diabetes.Curr Neuropharmacol. 2024;22(8):1327-1343. doi: 10.2174/1570159X22666240124164804. Curr Neuropharmacol. 2024. PMID: 38279738 Free PMC article. Review.
-
Using lickometry to infer differential contributions of salience network regions during compulsion-like alcohol drinking.Addict Neurosci. 2023 Sep;7:100102. doi: 10.1016/j.addicn.2023.100102. Epub 2023 May 4. Addict Neurosci. 2023. PMID: 38736902 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

