Oligodendrocytes enhance axonal energy metabolism by deacetylation of mitochondrial proteins through transcellular delivery of SIRT2
- PMID: 34506725
- PMCID: PMC8571020
- DOI: 10.1016/j.neuron.2021.08.011
Oligodendrocytes enhance axonal energy metabolism by deacetylation of mitochondrial proteins through transcellular delivery of SIRT2
Abstract
Neurons require mechanisms to maintain ATP homeostasis in axons, which are highly vulnerable to bioenergetic failure. Here, we elucidate a transcellular signaling mechanism by which oligodendrocytes support axonal energy metabolism via transcellular delivery of NAD-dependent deacetylase SIRT2. SIRT2 is undetectable in neurons but enriched in oligodendrocytes and released within exosomes. By deleting sirt2, knocking down SIRT2, or blocking exosome release, we demonstrate that transcellular delivery of SIRT2 is critical for axonal energy enhancement. Mass spectrometry and acetylation analyses indicate that neurons treated with oligodendrocyte-conditioned media from WT, but not sirt2-knockout, mice exhibit strong deacetylation of mitochondrial adenine nucleotide translocases 1 and 2 (ANT1/2). In vivo delivery of SIRT2-filled exosomes into myelinated axons rescues mitochondrial integrity in sirt2-knockout mouse spinal cords. Thus, our study reveals an oligodendrocyte-to-axon delivery of SIRT2, which enhances ATP production by deacetylating mitochondrial proteins, providing a target for boosting axonal bioenergetic metabolism in neurological disorders.
Keywords: acetylation; adenine nucleotide translocases 1 and 2; axonal ATP; axonal energetics; axonal mitochondria; energy metabolism; exosome; myelin; oligodendrocyte; sirtuin 2.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


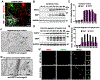


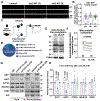
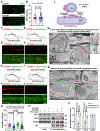
Comment in
-
Superfood for axons: Glial exosomes boost axonal energetics by delivery of SIRT2.Neuron. 2021 Nov 3;109(21):3397-3400. doi: 10.1016/j.neuron.2021.10.016. Neuron. 2021. PMID: 34735790
References
-
- Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Jacob Filho W, Lent R, and Herculano-Houzel S (2009). Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol 513, 532–541. - PubMed
-
- Black JA, and Waxman SG (1988). The perinodal astrocyte. Glia 1, 169–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

