Planned Granulocyte Colony-Stimulating Factor Adversely Impacts Survival after Allogeneic Hematopoietic Cell Transplantation Performed with Thymoglobulin for Myeloid Malignancy
- PMID: 34507002
- PMCID: PMC8671234
- DOI: 10.1016/j.jtct.2021.08.031
Planned Granulocyte Colony-Stimulating Factor Adversely Impacts Survival after Allogeneic Hematopoietic Cell Transplantation Performed with Thymoglobulin for Myeloid Malignancy
Abstract
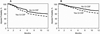
The in vivo depletion of recipient and donor T lymphocytes using antithymocyte globulin (ATG; Thymoglobulin) is widely adopted in allogeneic hematopoietic stem cell transplantation (HCT) to reduce the incidence of both graft failure and graft-versus-host disease (GVHD). However, excess toxicity to donor lymphocytes may hamper immune reconstitution, compromising antitumor effects and increasing infection. Granulocyte-colony stimulating factor (G-CSF) administered early after HCT may increase ATG-mediated lymphotoxicity. This study aimed to investigate the effect of an interaction between ATG and post-transplantation granulocyte colony-stimulating factor (G-CSF) on allogeneic HCT outcomes, using the Center for International Blood and Marrow Transplant Research (CIBMTR) registry. We studied patients age ≥18 years with acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS) who received Thymoglobulin-containing preparative regimens for HLA-matched sibling/unrelated or mismatched unrelated donor HCT between 2010 and 2018. The effect of planned G-CSF that was started between pretransplantation day 3 and post-transplantation day 12 was studied in comparison with transplantations that did not include G-CSF. Cox regression models were built to identify risk factors associated with outcomes at 1 year after transplantation. A total of 874 patients met the study eligibility criteria, of whom 459 (53%) received planned G-CSF. HCT with planned G-CSF was associated with a significantly increased risk for nonrelapse mortality (NRM) (hazard ratio [HR] 2.03; P <.0001; 21% versus 12%) compared to HCT without G-CSF. The 6-month incidence of viral infection was higher with G-CSF (56% versus 47%; P = .007), with a particular increase in Epstein-Barr virus infections (19% versus 11%; P = .002). The observed higher NRM with planned G-CSF led to lower overall survival (HR, 1.52; P = .0005; 61% versus 72%). There was no difference in GVHD risk between the treatment groups. We performed 2 subgroup analyses showing that our findings held true in patients age ≥50 years and in centers where G-CSF was used in some, but not all, patients. In allogeneic peripheral blood HCT performed with Thymoglobulin for AML and MDS, G-CSF administered early post-transplantation resulted in a 2-fold increase in NRM and a 10% absolute decrement in survival. The use of planned G-CSF in the early post-transplantation period should be carefully considered on an individual patient basis, weighing any perceived benefits against these risks.
Keywords: Antithymocyte globulin; Filgrastim; Granulocyte colony-stimulating factor; Hematopoietic stem cell transplantation; Thymoglobulin.
Copyright © 2021 The American Society for Transplantation and Cellular Therapy. All rights reserved.
Conflict of interest statement
FINANCIAL CONFLICT OF INTEREST
The authors declare none
Figures

Similar articles
-
Post-transplant Cyclophosphamide Versus Thymoglobulin in HLA-Mismatched Unrelated Donor Transplant for Acute Myelogenous Leukemia and Myelodysplastic Syndrome.Transplant Cell Ther. 2021 Sep;27(9):760-767. doi: 10.1016/j.jtct.2021.06.018. Epub 2021 Jun 23. Transplant Cell Ther. 2021. PMID: 34174469
-
Role of antithymocyte globulin and granulocyte-colony stimulating factor-mobilized bone marrow in allogeneic transplantation for patients with hematologic malignancies.Biol Blood Marrow Transplant. 2009 Feb;15(2):266-73. doi: 10.1016/j.bbmt.2008.11.029. Biol Blood Marrow Transplant. 2009. PMID: 19167687 Clinical Trial.
-
Low-Dose Antithymocyte Globulin for Graft-versus-Host-Disease Prophylaxis in Matched Unrelated Allogeneic Hematopoietic Stem Cell Transplantation.Biol Blood Marrow Transplant. 2017 Dec;23(12):2096-2101. doi: 10.1016/j.bbmt.2017.08.007. Epub 2017 Aug 15. Biol Blood Marrow Transplant. 2017. PMID: 28821454
-
Alloreactivity as therapeutic principle in the treatment of hematologic malignancies. Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning.Dan Med Bull. 2007 May;54(2):112-39. Dan Med Bull. 2007. PMID: 17521527 Review.
-
Haploidentical transplants with a G-CSF/ATG-based protocol: Experience from China.Blood Rev. 2023 Nov;62:101035. doi: 10.1016/j.blre.2022.101035. Epub 2022 Nov 15. Blood Rev. 2023. PMID: 36404244 Review.
Cited by
-
Impact of rabbit anti-thymocyte globulin (ATG) exposure on outcomes after ex vivo T-cell-depleted hematopoietic cell transplantation in pediatric and young adult patients.Cytotherapy. 2024 Apr;26(4):351-359. doi: 10.1016/j.jcyt.2024.01.004. Epub 2024 Feb 12. Cytotherapy. 2024. PMID: 38349310 Free PMC article.
-
Graft-versus-host disease: teaching old drugs new tricks at less cost.Front Immunol. 2023 Aug 3;14:1225748. doi: 10.3389/fimmu.2023.1225748. eCollection 2023. Front Immunol. 2023. PMID: 37600820 Free PMC article. Review.
-
Allogeneic haematopoietic cell transplants as dynamical systems: influence of early-term immune milieu on long-term T-cell recovery.Clin Transl Immunology. 2023 Jul 13;12(7):e1458. doi: 10.1002/cti2.1458. eCollection 2023. Clin Transl Immunology. 2023. PMID: 37457614 Free PMC article.
-
Impact of Granulocyte Colony-Stimulating Factor (G-CSF) on Clinical Outcomes in Allogeneic Hematopoietic Cell Transplantation: Does Speeding Up Neutrophil Engraftment Make a Difference?Transplant Direct. 2025 Jan 9;11(2):e1753. doi: 10.1097/TXD.0000000000001753. eCollection 2025 Feb. Transplant Direct. 2025. PMID: 39802196 Free PMC article.
-
Different impacts of granulocyte colony-stimulating factor administration on allogeneic hematopoietic cell transplant outcomes for adult acute myeloid leukemia according to graft type.Am J Hematol. 2025 Jan;100(1):66-77. doi: 10.1002/ajh.27521. Epub 2024 Nov 20. Am J Hematol. 2025. PMID: 39564683 Free PMC article.
References
-
- Bacigalupo A, Lamparelli T, Barisione G, et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transplant. 2006;12(5):560–565. - PubMed
-
- Bonifazi F, Solano C, Wolschke C, et al. Acute GVHD prophylaxis plus ATLG after myeloablative allogeneic haemopoietic peripheral blood stem-cell transplantation from HLA-identical siblings in patients with acute myeloid leukaemia in remission: final results of quality of life and long-term outcome analysis of a phase 3 randomised study. Lancet Haematol. 2019;6(2):e89–e99. - PubMed
-
- Finke J, Schmoor C, Bethge WA, et al. Long-term outcomes after standard graft-versus-host disease prophylaxis with or without anti-human-T-lymphocyte immunoglobulin in haemopoietic cell transplantation from matched unrelated donors: final results of a randomised controlled trial. Lancet Haematol. 2017;4(6):e293–e301. - PubMed
-
- Soiffer RJ, Kim HT, McGuirk J, et al. Prospective, Randomized, Double-Blind, Phase III Clinical Trial of Anti-T-Lymphocyte Globulin to Assess Impact on Chronic Graft-Versus-Host Disease-Free Survival in Patients Undergoing HLA-Matched Unrelated Myeloablative Hematopoietic Cell Transplantation. J Clin Oncol. 2017;35(36):4003–4011. - PMC - PubMed
-
- Walker I, Panzarella T, Couban S, et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: a randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol. 2016;17(2):164–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

