Alterations in corticotropin-releasing factor receptor type 1 in the preoptic area and hypothalamus in mice during the postpartum period
- PMID: 34507241
- PMCID: PMC8653990
- DOI: 10.1016/j.yhbeh.2021.105044
Alterations in corticotropin-releasing factor receptor type 1 in the preoptic area and hypothalamus in mice during the postpartum period
Abstract
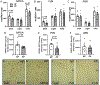
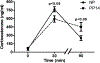
Corticotropin-releasing factor (CRF) signaling through CRF receptor 1 (CRFR1) regulates autonomic, endocrine, and behavioral responses to stress, as well as behavioral changes during the maternal period. Previous work in our lab reported higher levels of CRFR1 in female, compared to male, mice within the rostral anteroventral periventricular nucleus (AVPV/PeN), a brain region involved in maternal behaviors. In this study, we used CRFR1-GFP reporter mice to investigate whether the reproductive status (postpartum vs. nulliparous) of acutely stressed females affects levels of CRFR1 in the AVPV/PeN and other regions involved in maternal functions. Compared to nulliparous, postpartum day 14 females showed increased AVPV/PeN CRFR1-GFP immunoreactivity and an elevated number of restraint stress-activated AVPV/PeN CRFR1 cells as assessed by immunohistochemical co-localization of CRFR1-GFP and phosphorylated CREB (pCREB). The medial preoptic area (MPOA) and paraventricular hypothalamus (PVN) of postpartum mice showed modest decreases in CRFR1-GFP immunoreactivity, while increased CRFR1-GFP/pCREB co-expressing cells were found in the PVN following restraint stress relative to nulliparous mice. Tyrosine hydroxylase (TH) and CRFR1-GFP co-localization was also assessed in the AVPV/PeN and other regions and revealed a decrease in co-localized neurons in the AVPV/PeN and ventral tegmental area of postpartum mice. Corticosterone analysis of restrained mice revealed blunted peak, but elevated recovery, levels in postpartum compared to nulliparous mice. Finally, we investigated projection patterns of AVPV/PeN CRFR1 neurons using female CRFR1-Cre mice and revealed dense efferent projections to several preoptic, hypothalamic, and hindbrain regions known to control stress-associated and maternal functions. Together, these findings contribute to our understanding of the neurobiology that might underlie changes in stress-related functions during the postpartum period.
Keywords: Corticotropin-releasing factor; Corticotropin-releasing factor receptor type 1; Hypothalamus; Paraventricular nucleus; Postpartum; Preoptic area; Rostral anteroventral periventricular nucleus; Sex differences; Tyrosine hydroxylase.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest
None declared.
Figures





References
-
- Agrati D, Browne D, Jonas W, Meaney M, Atkinson L, Steiner M, Fleming AS, 2015. Maternal anxiety from pregnancy to 2 years postpartum: transactional patterns of maternal early adversity and child temperament. Arch Womens Ment Health 18, 693–705. - PubMed
-
- Altemus M, Deuster PA, Galliven E, Carter CS, & Gold PW, 1995. Suppression of hypothalmic-pituitary-adrenal axis responses to stress in lactating women. The Journal of Clinical Endocrinology & Metabolism, 80(10), 2954–2959. - PubMed
-
- Barnett B, Schaafsma MF, Guzman AM and Parker GB, 1991. Maternal anxiety: a 5-year review of an intervention study. Journal of Child Psychology and Psychiatry, 32(3), 423–438. - PubMed
-
- Berghorn KA, Le WW, Sherman TG and Hoffman GE, 2001. Suckling stimulus suppresses messenger RNA for tyrosine hydroxylase in arcuate neurons during lactation. Journal of Comparative Neurology, 438(4), 423–432. - PubMed
-
- Brand SR and Brennan PA, 2009. Impact of antenatal and postpartum maternal mental illness: how are the children? Clinical obstetrics and gynecology, 52(3), 441–455. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

