A biofabrication method to align cells within bioprinted photocrosslinkable and cell-degradable hydrogel constructs via embedded fibers
- PMID: 34507304
- PMCID: PMC8603602
- DOI: 10.1088/1758-5090/ac25cc
A biofabrication method to align cells within bioprinted photocrosslinkable and cell-degradable hydrogel constructs via embedded fibers
Abstract
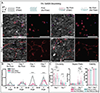
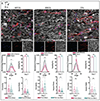
The extracellular matrix (ECM) is composed of biochemical and biophysical cues that control cell behaviors and bulk mechanical properties. For example, anisotropy of the ECM and cell alignment are essential in the directional properties of tissues such as myocardium, tendon, and the knee meniscus. Technologies are needed to introduce anisotropic behavior into biomaterial constructs that can be used for the engineering of tissues as models and towards translational therapies. To address this, we developed an approach to align hydrogel fibers within cell-degradable bioink filaments with extrusion printing, where shear stresses during printing align fibers and photocrosslinking stabilizes the fiber orientation. Suspensions of hydrogel fibers were produced through the mechanical fragmentation of electrospun scaffolds of norbornene-modified hyaluronic acid, which were then encapsulated with meniscal fibrochondrocytes, mesenchymal stromal cells, or cardiac fibroblasts within gelatin-methacrylamide bioinks during extrusion printing into agarose suspension baths. Bioprinting parameters such as the needle diameter and the bioink flow rate influenced shear profiles, whereas the suspension bath properties and needle translation speed influenced filament diameters and uniformity. When optimized, filaments were formed with high levels of fiber alignment, which resulted in directional cell spreading during culture over one week. Controls that included bioprinted filaments without fibers or non-printed hydrogels of the same compositions either with or without fibers resulted in random cell spreading during culture. Further, constructs were printed with variable fiber and resulting cell alignment by varying print direction or using multi-material printing with and without fibers. This biofabrication technology advances our ability to fabricate constructs containing aligned cells towards tissue repair and the development of physiological tissue models.
Keywords: alignment; anisotropy; bioprinting; fibers; hydrogel.
© 2021 IOP Publishing Ltd.
Figures
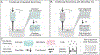





References
-
- Datta P, Vyas V, Dhara S, Chowdhury AR and Barui A 2019. Anisotropy Properties of Tissues: A Basis for Fabrication of Biomimetic Anisotropic Scaffolds for Tissue Engineering Journal of Bionic Engineering 16 842–68
-
- Peng R, Yao X and Ding J 2011. Effect of cell anisotropy on differentiation of stem cells on micropatterned surfaces through the controlled single cell adhesion Biomaterials 32 8048–57 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
