18F-FDG-PET-MRI for the assessment of acute intestinal graft-versus-host-disease (GvHD)
- PMID: 34507549
- PMCID: PMC8434740
- DOI: 10.1186/s12885-021-08748-x
18F-FDG-PET-MRI for the assessment of acute intestinal graft-versus-host-disease (GvHD)
Abstract
Background: Graft versus host disease (GvHD) is a frequent complication of allogeneic stem cell transplantation (alloSCT), significantly increasing mortality. Previous imaging studies focused on the assessment of intestinal GvHD with contrast-enhanced MRI/CT or 18F-FDG-PET imaging alone. The objective of this retrospective study was to elucidate the diagnostic value of a combined 18F-FDG-PET-MRI protocol in patients with acute intestinal GvHD.
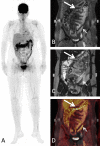
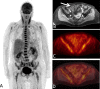
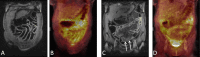
Methods: Between 2/2015 and 8/2019, 21 patients with acute intestinal GvHD underwent 18F-FDG-PET-MRI. PET, MRI and PET-MRI datasets were independently reviewed. Readers assessed the number of affected segments of the lower gastrointestinal tract and the reliability of the diagnosis on a 5-point Likert scale and quantitative PET (SUVmax, SUVpeak, metabolic volume (MV)) and MRI parameter (wall thickness), were correlated to clinical staging of acute intestinal GvHD.
Results: The detection rate for acute intestinal GvHD was 56.8% for PET, 61.4% for MRI and 100% for PET-MRI. PET-MRI (median Likert-scale value: 5; range: 4-5) offers a significantly higher reliability of the diagnosis compared to PET (median: 4; range: 2-5; p = 0.01) and MRI alone (median: 4; range: 3-5; p = 0.03). The number of affected segments in PET-MRI (rs = 0.677; p < 0.001) and the MV (rs = 0.703; p < 0.001) correlated significantly with the clinical stage. SUVmax (rs = 0.345; p = 0.14), SUVpeak (rs = 0.276; p = 0.24) and wall thickening (rs = 0.174; p = 0.17) did not show a significant correlation to clinical stage.
Conclusion: 18F-FDG-PET-MRI allows for highly reliable assessment of acute intestinal GvHD and adds information indicating clinical severity.
Keywords: FDG; GvHD; Inflammation; PET-MRI.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, Chanswangphuwana C, Efebera YA, Holler E, Litzow M, Ordemann R, Qayed M, Renteria AS, Reshef R, Wölfl M, Chen YB, Goldstein S, Jagasia M, Locatelli F, Mielke S, Porter D, Schechter T, Shekhovtsova Z, Ferrara JLM, Levine JE. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai acute GVHD international consortium. Biol Blood Marrow Transplant. 2016;22(1):4–10. doi: 10.1016/j.bbmt.2015.09.001. - DOI - PMC - PubMed
-
- Scott AP, Tey S-K, Butler J, Kennedy GA. Diagnostic utility of endoscopy and biopsy in suspected acute gastrointestinal graft-versus-host disease after hematopoietic progenitor cell transplantation. Biol blood marrow Transplant J Am Soc Blood Marrow Transplant. 2018;24(6):1294–1298. doi: 10.1016/j.bbmt.2018.01.034. - DOI - PubMed
-
- Neurath MF, Vehling D, Schunk K, Holtmann M, Brockmann H, Helisch A, Orth T, Schreckenberger M, Galle PR, Bartenstein P. Noninvasive assessment of Crohn’s disease activity: a comparison of 18F-fluorodeoxyglucose positron emission tomography, hydromagnetic resonance imaging, and granulocyte scintigraphy with labeled antibodies. Am J Gastroenterol. 2002;97(8):1978–1985. doi: 10.1016/S0002-9270(02)04192-8. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

