PEGylated recombinant human hyaluronidase (PEGPH20) pre-treatment improves intra-tumour distribution and efficacy of paclitaxel in preclinical models
- PMID: 34507591
- PMCID: PMC8434701
- DOI: 10.1186/s13046-021-02070-x
PEGylated recombinant human hyaluronidase (PEGPH20) pre-treatment improves intra-tumour distribution and efficacy of paclitaxel in preclinical models
Abstract
Background: Scarce drug penetration in solid tumours is one of the possible causes of the limited efficacy of chemotherapy and is related to the altered tumour microenvironment. The abnormal tumour extracellular matrix (ECM) together with abnormal blood and lymphatic vessels, reactive stroma and inflammation all affect the uptake, distribution and efficacy of anticancer drugs.
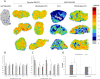
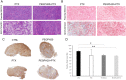
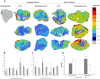
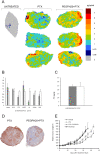
Methods: We investigated the effect of PEGylated recombinant human hyaluronidase PH20 (PEGPH20) pre-treatment in degrading hyaluronan (hyaluronic acid; HA), one of the main components of the ECM, to improve the delivery of antitumor drugs and increase their therapeutic efficacy. The antitumor activity of paclitaxel (PTX) in HA synthase 3-overexpressing and wild-type SKOV3 ovarian cancer model and in the BxPC3 pancreas xenograft tumour model, was evaluated by monitoring tumour growth with or without PEGPH20 pre-treatment. Pharmacokinetics and tumour penetration of PTX were assessed by HPLC and mass spectrometry imaging analysis in the same tumour models. Tumour tissue architecture and HA deposition were analysed by histochemistry.
Results: Pre-treatment with PEGPH20 modified tumour tissue architecture and improved the antitumor activity of paclitaxel in the SKOV3/HAS3 tumour model, favouring its accumulation and more homogeneous intra-tumour distribution, as assessed by quantitative and qualitative analysis. PEGPH20 also reduced HA content influencing, though less markedly, PTX distribution and antitumor activity in the BxPC3 tumour model.
Conclusion: Remodelling the stroma of HA-rich tumours by depletion of HA with PEGPH20 pre-treatment, is a potentially successful strategy to improve the intra-tumour distribution of anticancer drugs, increasing their therapeutic efficacy, without increasing toxicity.
Keywords: Drug distribution; Extracellular matrix; Hyaluronan; Mass spectrometry imaging; Solid tumours.
© 2021. The Author(s).
Conflict of interest statement
David W. Kang is an employee, owns stock and has stock options in Halozyme Therapeutics. Barbara Blouw was an employee, owned stock and had stock options in Halozyme Therapeutics at the time of the study.
Figures

References
-
- Lankelma J, Dekker H, Luque FR, Luykx S, Hoekman K, van der Valk P, et al. Doxorubicin gradients in human breast cancer. Clin Cancer Res. 1999;5(7):1703-7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

