Exogenous Aβ seeds induce Aβ depositions in the blood vessels rather than the brain parenchyma, independently of Aβ strain-specific information
- PMID: 34507620
- PMCID: PMC8431898
- DOI: 10.1186/s40478-021-01252-0
Exogenous Aβ seeds induce Aβ depositions in the blood vessels rather than the brain parenchyma, independently of Aβ strain-specific information
Abstract
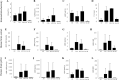
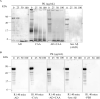
Little is known about the effects of parenchymal or vascular amyloid β peptide (Aβ) deposition in the brain. We hypothesized that Aβ strain-specific information defines whether Aβ deposits on the brain parenchyma or blood vessels. We investigated 12 autopsied patients with different severities of Aβ plaques and cerebral amyloid angiopathy (CAA), and performed a seeding study using an Alzheimer's disease (AD) mouse model in which brain homogenates derived from the autopsied patients were injected intracerebrally. Based on the predominant pathological features, we classified the autopsied patients into four groups: AD, CAA, AD + CAA, and less Aβ. One year after the injection, the pathological and biochemical features of Aβ in the autopsied human brains were not preserved in the human brain extract-injected mice. The CAA counts in the mice injected with all four types of human brain extracts were significantly higher than those in mice injected with PBS. Interestingly, parenchymal and vascular Aβ depositions were observed in the mice that were injected with the human brain homogenate from the less Aβ group. The Aβ and CAA seeding activities, which had significant positive correlations with the Aβ oligomer ratio in the human brain extracts, were significantly higher in the human brain homogenate from the less Aβ group than in the other three groups. These results indicate that exogenous Aβ seeds from different Aβ pathologies induced Aβ deposition in the blood vessels rather than the brain parenchyma without being influenced by Aβ strain-specific information, which might be why CAA is a predominant feature of Aβ pathology in iatrogenic transmission cases. Furthermore, our results suggest that iatrogenic transmission of Aβ pathology might occur due to contamination of brain tissues from patients with little Aβ pathology, and the development of inactivation methods for Aβ seeding activity to prevent iatrogenic transmission is urgently required.
Keywords: Alzheimer’s disease; Amyloid β peptide; Cerebral amyloid angiopathy; Iatrogenic; Strain; Transmission.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Albargothy NJ, Johnston DA, MacGregor-Sharp M, Weller RO, Verma A, Hawkes CA, Carare RO. Convective influx/glymphatic system: tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways. Acta Neuropathol. 2018;136:139–152. doi: 10.1007/s00401-018-1862-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

